For ideal gases, the development of the constant-volume specific heat yields Prove this by using the definitions
Question:
For ideal gases, the development of the constant-volume specific heat yields
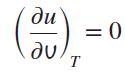
Prove this by using the definitions of pressure and temperature, T = (∂u/∂s)v and P = −(∂u/∂v)s.
Transcribed Image Text:
ди = 0 du.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
It is to be proven by using the definitions of ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Question Posted:
Students also viewed these Engineering questions
-
For ideal gases, the development of the constant-pressure specific heat yields Prove this by using the definitions of pressure and temperature, T = (u/s)v and P = - (u/v)s. ah aP
-
Plss answer the highlighted number SERVATION OF MASS AND ENERGY unknown flow rate; however, the water temperature increases from 13C to 24 C. Also, it is known that 1 kg of water will absorb 4.2 kJ...
-
14 Thermodynamics and Thermochemistry . The reaction, MgO(s) + C(s) Mg(s) + CO(g ) 18 The entropy change associated with the conversion of 1 kg of ice at 273 K to water vapours at 383 K is for which...
-
Route Canal Shipping Company has the following schedule for aging of accounts receivable: AGE OF RECEIVABLES APRIL 30, 2001 a. Fill in column (4) for each month. b. If the firm had $1,440,000 in...
-
The paired data below consist of weights (in kilograms) of discarded paper and sizes of the households. Paper Household size 2 1.09 3.43 4.33 4.00 3.96 3.16 3.10 5.18
-
Let us consider again the sure lottery \(a_{1}\), which guarantees a payoff with probability one, and lottery \(a_{2}\), obtained by the mean-preserving spread , featuring equally likely outcomes and...
-
What might account for the fact that, on average, the investor return provided by the firms of endearment exceeded that of the Standard and Poors and Dow Jones averages?
-
Assume that Coaltown Corporation has a machine that cost $52,000, has a book value of $35,000, and has a market value of $40,000. The machine is used in Coaltowns manufacturing process. For each of...
-
Microsoft Corporation makes Xbox video game consoles. For Microsoft's financial year YYYY, please make use of the following provided information regarding the inventory of those Xbox consoles:...
-
The cellphone brands owned by a sample of 20 respondents were: Apple, Samsung, Appel, Nokia, Blackberry, HTC, Apple, Samsung, HTC, LG, Blueberry, Samsung, Samsung, APPLE, Motorola, Apple, Samsung,...
-
Develop expressions for h, u, s, P r , and v r for an ideal gas whose c o p is given by where a i , a 0 , n, and are empirical constants. c = Ea,T- BIT eNT - 1
-
Estimate the c p of nitrogen at 300 kPa and 400 K, using (a) The relation in Prob. 1291, Data From Q#91: Consider an infinitesimal reversible adiabatic compression or expansion process. By taking s =...
-
Suppose we have samples of five men and of five women and have conducted a randomization test to compare the sexes on the variable Y = pulse. Further, suppose we have found that in 120 out of the 252...
-
A jet of length L = 18 m is travelling at V = 400 m/s at an altitude of h = 6 km. At that altitude, the speed of sound is c = 316 m/s, the density of air is p = 0.6597 kg/m, and the viscosity is =...
-
Determine the moment of inertia with respect to x axis 12 mm 8 mm- y O 12 mm - 24 mm 24 mm Fig. P9.31 and P9.33 6 mm 24 mm 24 mm 6 mm x
-
Determine the forces in members BC, BE, and EF. (15P) 4 m 4 m 4 m 5 KN 10 kN 10 kN 5 kN E
-
Example 1.5 :Four masses 150 kg, 200 kg, 100 kg and 250 kg are attached to a shaft revolving at radii 150 mm, 200 mm, 100 mm and 250 mm; in planes A, B, C and D respectively. The planes B, C and D...
-
Problem 1: Air moves over flat plate with a uniform free stream velocity of 10 m/s. At a position 15 cm away from the front edge of the plate, what is the boundary layer thickness? Use parabolic...
-
What are some obstacles to companies adopting a supply-chain approach?
-
An environmentalist wants to determine if the median amount of potassium (mg/L) in rainwater in Lincoln County, Nebraska, is different from that in the rainwater in Clarendon County, South Carolina....
-
It is proposed to run a thermoelectric generator in conjunction with a solar pond that can supply heat at a rate of 7 106 kJ/h at 90oC. The waste heat is to be rejected to the environment at 22oC....
-
A typical 200-m2 house can be cooled adequately by a 3.5-ton air conditioner whose COP is 4.0. Determine the rate of heat gain of the house when the air conditioner is running continuously to...
-
Consider a steady-flow Carnot refrigeration cycle that uses refrigerant-134a as the working fluid. The maximum and minimum temperatures in the cycle are 30 and - 20oC, respectively. The quality of...
-
What are the main role of a businessmen to build the good business relation and communication to make the business better everyday?
-
Let's assume your lab balloons, when filled with air, each had a mass of 3.00 grams. In a variation of your lab activity, you attach one of these balloons to a string such that the distance from the...
-
Samantha normally requires 1 3 7 0 0 kJ ( about 3 2 7 4 Calories ) of food energy per day. If Samantha consumes 1 4 3 8 5 kJ per day, she will steadily gain weight. How much time must Samantha spend...

Study smarter with the SolutionInn App


