Use the Gibbs function to determine the equilibrium constant of the H 2 O H 2
Question:
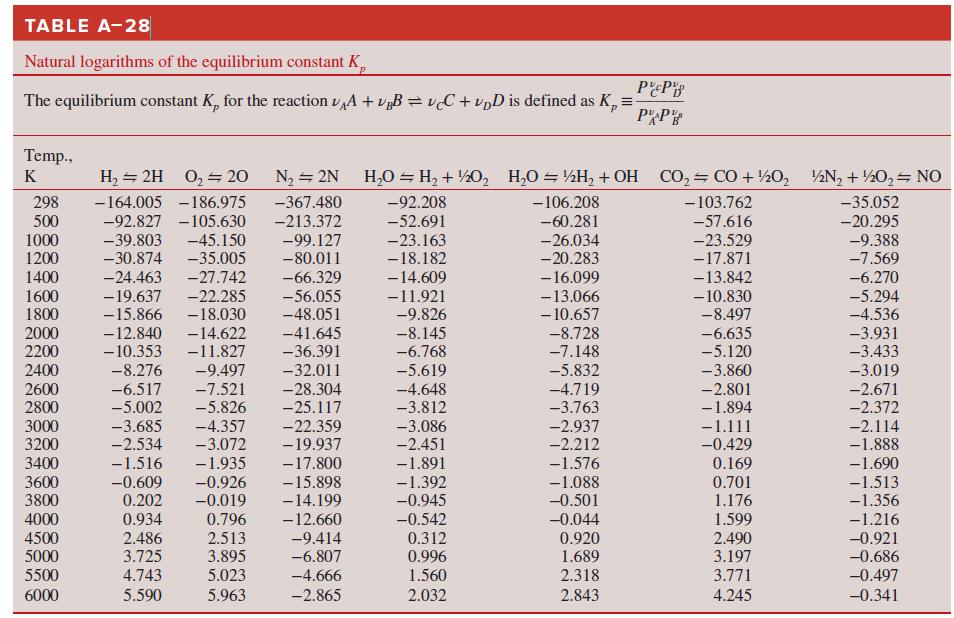
Use the Gibbs function to determine the equilibrium constant of the H2O ⇌ H2 + 1/2 O2 reaction at
(a) 1440 R
(b) 3960 R.
How do these compare to the equilibrium constants of Table A–28?
Transcribed Image Text:
TABLE A-28 Natural logarithms of the equilibrium constant K, The equilibrium constant K, for the reaction vA + v„B = vC + vpD is defined as K, = PP Temp., K H, = 2H 0, 20 N2 = 2N H,0 = H, + ½0, H,O = ½H, + OH CO, CO + ½0, ½N, + ½0, NO 298 500 1000 -164.005 -186.975 -367.480 -92.208 -106.208 - 103.762 -35.052 -92.827 -105.630 -45.150 -35.005 -27.742 -213.372 -52.691 -60.281 -57.616 -20.295 -39.803 -30.874 -99.127 -80.011 -9.388 -7.569 -23.163 -26.034 -20.283 -23.529 -17.871 -13.842 1200 -18.182 -6.270 -5.294 1400 -24.463 -66.329 -14.609 1600 1800 -19.637 -15.866 -22.285 -18.030 -14.622 - 16.099 -13.066 - 10.657 -56.055 -48.051 -11.921 -9.826 - 10.830 -8.497 -6.635 -5.120 -4.536 2000 2200 -12.840 - 10.353 -41.645 -8.145 -6.768 -8.728 -7.148 -3.931 -3.433 -11.827 -36.391 2400 -8.276 -9.497 -32.011 -5.619 -4.648 -3.812 -5.832 -3.860 -3.019 -28.304 2600 2800 -6.517 -5.002 -7.521 -5.826 -4.719 -3.763 -2.801 -1.894 -2.671 -2.372 -25.117 -22.359 3000 3200 -3.685 -2.534 -4.357 -3.072 -3.086 -2.937 -2.212 -1.111 -0.429 -2.114 -1.888 -19.937 -2.451 3400 -1.516 -1.935 -17.800 -1.891 -1.576 0.169 3600 3800 -0.609 0.202 -0.926 -0.019 -15.898 -14.199 -12.660 -1.690 -1.513 -1.356 -1.392 -0.945 -1.088 -0.501 0.701 1.176 0.796 2.513 3.895 0.934 -0.542 -0.044 0.920 1.689 4000 1.599 -1.216 4500 5000 2.486 3.725 -9.414 -6.807 0.312 2.490 3.197 -0.921 -0.686 0.996 5500 4.743 5.023 -4.666 1.560 2.318 3.771 -0.497 6000 5.590 5.963 -2.865 2.032 2.843 4.245 -0.341
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
The equilibrium constant of the reaction is to be dete...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Question Posted:
Students also viewed these Engineering questions
-
The equilibrium constant for the reaction H2 + at 1 atm and 1500C is given to be K. Of the reactions given below, all at 1500C, the reaction that has a different equilibrium constant is (a) H2 + 12O2...
-
The equilibrium constant for the H2 + ½ O2 H2O reaction at 1 atm and 1200 K is KP. Use this information to determine the equilibrium constant for the following reactions: (a) at l atm H, +...
-
The table below shows data (from a 2004 Bureau of the Census report) on the number of times 20- to 24-year-old men have been married. a. Verify that the mean number of times men have been married is...
-
Consider a portfolio of the following derivatives where the counterparty is an OECD bank. Derivative 8-year interest rate swap 6-month option on an equity 1-year swap on precious metals 9-month...
-
Digits (0, 1, 2,. . . ,9) are randomly selected for telephone numbers in surveys. The random variable x is the selected digit. a. Find the mean and standard deviation of x. b. Find the z score for...
-
Why must a CPA practicing tax be ethical when providing tax advice to others?
-
Two parallel-plate capacitors have the same plate separation. The plate area of capacitor 1 is twice that of capacitor 2. (a) How do the potential differences across the two capacitors compare if the...
-
The following data relate the sales figures of the bar in Mark Kaltenbachs small bed- and- breakfast inn in Portand, to the number of guests registered that week: a) Perform a linear regression that...
-
6. An igloo, a hemispherical enclosure built of ice (1.67 J/m-sC), has as inner radius of 2.50m. The thickness of the ice is 0.5m. At what rate must thermal energy be generated to maintain the air...
-
Kate Stephens, the COO of BioDerm, has asked her cost management team for a product-line profitability analysis for her company's two products, Xderm and Yderm. The two skin-care products require a...
-
A mixture of ideal gases consists of the following gases by mole fraction: 10 percent CO 2 , 60 percent H 2 O, and 30 percent CO. Determine the Gibbs function of the CO in this mixture when the...
-
An inventor claims she can produce hydrogen gas by the reversible reaction 2H 2 O H 2 + O 2 . Determine the mole fractions of the hydrogen and oxygen produced when this reaction occurs at 4000 K and...
-
Preferably without consulting reference material, draw a periodic table and indicate the elements that form saline, metallic, and metalloid carbides.
-
what pension and plan would you advice for a self - employed 3 6 year old who has 2 4 5 0 0 taxable income and wants to retire in 1 8 years?
-
What are three (3) important features of a circulation system and technology within a public library, and also an educational institution library?
-
You are the owner of FAST DELIVERY that provides delivery services. Assume your September 2 0 2 1 revenue is $ 1 5 , 0 0 0 and your operating expenses are $ 7 , 2 0 0 and include rent $ 1 , 2 0 0 ;...
-
Two charges q1 = 3.0 C and 92 = - 2.0 C are separated by 10 cm. How far to the right of q2 on the line connecting the two charges is the electric field zero?
-
What is the major difference between liberals of the 18th century and liberals of today? explain
-
In what ways is Google prepared to respond to both organizational opportunities and organizational threats in the U.S. market?
-
As long as we can't lose any money, we have a risk-free investment." Discuss this comment. Q2: Both investing and gambling can be defined as "undertaking risk in order to earn a profit." Explain how...
-
Octane gas (C8H18) at 25oC is burned steadily with 30 percent excess air at 25oC, 1 atm, and 60 percent relative humidity. Assuming combustion is complete and adiabatic, calculate the exit...
-
Reconsider Prob. 15-75. Using EES (or other) software, investigate the effect of the relative humidity on the exit temperature of the product gases. Plot the exit temperature of the product gases as...
-
A coal from Pennsylvania has an ultimate analysis (by mass) as 84.36 percent C, 1.89 percent H2, 4.40 percent O2, 0.63 percent N2, 0.89 percent S, and 7.83 percent ash (non-combustibles) is burned in...
-
The function P(x)=0.35x-73 models the relationship between th e number of pretzels x that a certain vendor sells and the profit the vendor makes. Find P(1,000), the profit the vendor makes from...
-
Thermal Rising, Incorporated, makes paragliders for sale through specialty sporting goods stores. The company has a standard paraglider model, but also makes custom-designed paragliders. Management...
-
Klumper Corporation is a diversified manufacturer of industrial goods. The company's activity-based costing system contains the following six activity cost pools and activity rates: Activity Cost...

Study smarter with the SolutionInn App


