The electron in a hydrogen atom can be considered to be in a circular orbit with a
Question:
Problem 32.57
Electromagnetic radiation is emitted by accelerating charges. The rate at which energy is emitted from an accelerating charge that has charge q and acceleration a is given by
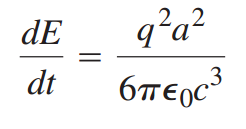
where c is the speed of light.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

University Physics with Modern Physics
ISBN: 978-0321696861
13th edition
Authors: Hugh D. Young, Roger A. Freedman, A. Lewis Ford
Question Posted:





