Ten mL of pure liquid water in a cylinder with a movable piston is heated at a
Question:
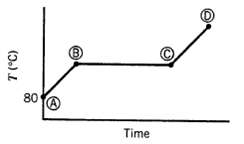
Ten mL of pure liquid water in a cylinder with a movable piston is heated at a constant pressure of 1 atm from an initial temperature of 80?C. The temperature of the system is monitored, and the following behavior is observed:
(a) What is happening in steps AB, BC, and CD? What is the temperature corresponding to the horizontal portion of the curve?
(b) Estimate the volume occupied by the water at points B and C. (Assume the vapor follows the ideal gas equation of state.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau
Question Posted:





