The Clausius-Mossotti equation (prob. 4.38) tells you how to calculate the susceptibility of a nonpolar substance, in
Question:
The Clausius-Mossotti equation (prob. 4.38) tells you how to calculate the susceptibility of a nonpolar substance, in terms of the atomic polarizability a. The Langevin equation tells you how to calculate the susceptibility of a polar substance, in terms of the permanent molecular dipole moment p. Here's how it goes:
(a) The energy of a dipole in an external field E is u = ?? p ?? E (Eq. 4.6); it ranges from -pE to +pE, depending on the orientation. Statistical mechanics says that for a material in equilibrium at absolute temperature T, the probability of a given molecule having energy u is proportional to the Boltzmann factor, exp (?? u/kT). The average energy of the dipoles is therefore where the integrals run from -pE to +pE. Use this to show that the polarization of a substance containing N molecules per unit volume is P = Np[coth(pE/kT) ?? (kT/pE)]. (4.73) That's the Langevin formula. Sketch P/Np as a function of pE/kT.
(b) Notice that for large fields/low temperatures, virtually all the molecules are lined up, and the material is nonlinear. Ordinarily, however, kT is much greater than pE. Show that in this r6gime the material is linear, and calculate its susceptibility, in terms of N, p, T, and k. Compute the susceptibility of water at 20o C, and compare the experimental value in Table 4.2. (The dipole moment of water is 6.1 x 10??30 C.m.) This is rather far off, because we have again neglected the distinction between E and Eelse. The agreement is better in low-density gases, for which the difference between E and Eelse is negligible. Try it for water vapor at 100o and 1atm.
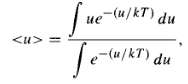
Step by Step Answer:






