The exit gas from an alcohol fermenter consists of an air-C02 mixture containing 10 mol% C02 that
Question:
The exit gas from an alcohol fermenter consists of an air-C02 mixture containing 10 mol% C02 that is to be absorbed in a 5.0-N solution of triethanolamine, containing 0.04 mol of carbon dioxide per mole of amine solution. If the column operates isothermally at 25oC, if the exit liquid contains 78.4% of the C02 in the feed gas to the absorber, and if absorption is carried out in a six-theoretical-plate column, calculate:(a) Moles of amine solution required per mole of feed gas.(b) Exit gas composition.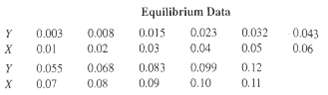 Y = moles C02/mole air; X = moles C02/mole aminesolution
Y = moles C02/mole air; X = moles C02/mole aminesolution
Transcribed Image Text:
Equilibrium Data 0.032 0.05 0.12 0.008 0.023 0.003 0.043 0.015 0.04 0.06 0.02 0.03 0.083 0.01 0.055 0.07 0.068 0.099 0.10 х 0.09 0.11 0.08
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (21 reviews)
Use the nomenclature and type of plot shown in Fig a Therefore for CO 2 b There...View the full answer

Answered By

Atuga Nichasius
I am a Highly skilled Online Tutor has a Bachelor’s Degree in Engineering as well as seven years of experience tutoring students in high school, bachelors and post graduate levels. I have a solid understanding of all learning styles as well as using asynchronous online platforms for tutoring needs. I individualise tutoring for students according to content tutoring needs assessments.
My strengths include good understanding of all teaching methods and learning styles and I am able to convey material to students in an easy to understand manner. I can also assists students with homework questions and test preparation strategies and I am able to help students in math, gre, business , and statistics
I consider myself to have excellent interpersonal and assessment skills with strong teaching presentation verbal and written communication
I love tutoring. I love doing it. I find it intrinsically satisfying to see the light come on in a student's eyes.
My first math lesson that I taught was when I was 5. My neighbor, still in diapers, kept skipping 4 when counting from 1 to 10. I worked with him until he could get all 10 numbers in a row, and match them up with his fingers.
My students drastically improve under my tutelage, generally seeing a two grade level improvement (F to C, C to A, for example), and all of them get a much clearer understanding!
I am committed to helping my students get the top grades no matter the cost. I will take extra hours with you, repeat myself a thousand times if I have to and guide you to the best of my ability until you understand the concept that I'm teaching you.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
Exit gas from an amination reactor contains 10 mole% ammonia (NH 3 ) vapor in a nitrogen (N 2 ) carrier gas. This gas mixture is fed into the bottom of a packed tower at a molar flow rate of 2.0...
-
The back of a framed picture that is to be hung is shown at the top of the next page. A nail is to be hammered into the wall, and the picture will be hung by the wire on the nail. a) If the center of...
-
A program file contains data that is to be used by various programs. True of False
-
Give examples of three exceptions to the Cardozo rule of foreseeability.
-
The unadjusted trial balance of Fashion Centre Ltd. contained the following accounts at November 30, the company's fiscal year end: Additional information and adjustment data: 1. The 12-month...
-
Enediynes are a class of compounds that include some antibiotic drugs. Draw the structure of an "enediyne" fragment that contains six carbons in a row. (Hint: di means "two.")
-
In the case of the previous question, if the effect of the finite speed of sound were neglected, would \(h\) be larger or smaller? What percentage error would be made? Previous question: One wants to...
-
This case will enable you to practice conducting planning and substantive analytical procedures for accounts in the acquisition and payment cycle. When analyzing the financial data, you may assume...
-
24. A water jet whose cross section area is a striker wall making an angle e with the normal and rebounds elastically. The velocity of water of density d is v. Force exerted on wall is :- 0 (1) 2avd...
-
Under fair-value accounting for an equity investment. which of the following affects the income the investor recognizes from its ownership of the investee? a. The investee's reported income adjusted...
-
Prove by equations why, in general, absorbers should be operated at high pressure and low temperature, while strippers should be operated at low pressure and high temperature. Also prove, by...
-
Ninety-five percent of the acetone vapor in an 85 vol% air stream is to be absorbed by countercurrent contact with pure water in a valve-tray column with an expected overall tray efficiency of 50%....
-
Mary Webster owns and manages a company that provides trenching services. Her clients are companies that need to lay power lines, gas lines, and fiber optic cable. Because trenching machines require...
-
What role does data locality optimization play in enhancing the performance of batch processing jobs, and how do techniques such as data partitioning and co-location influence data access patterns...
-
Given the function, Compute the following limits. (a) lim g (y) 9(y)= (b) lim g (y) (y+5 if y
-
Seemore Lens Company (SLC) sells contact lenses FOB destination. For the year ended December31, the company reported Inventory of $86,000 and Cost of Goods Sold of $452,000. Included in Inventory...
-
1) How can the below banking segments position themselves in order to maximize profits given the current situation of rising interest rates: a) The non-retail banking segment b) Retail banking...
-
Crystal had her money invested in two types of mutual funds: $2,900 in low-risk funds and $1,200 in high-risk funds. If the value of her high-risk funds grew by 35.00%, and that of the low-risk funds...
-
You establish the following details in connection with the non-current assets of Charlottes business for the year ended 31 December 2024: What figure for payments to acquire non-current assets will...
-
The following selected accounts and normal balances existed at year-end. Notice that expenses exceed revenue in this period. Make the four journal entries required to close the books: Accounts...
-
Why do scholars suggest that we are unlikely to see a convergence of HRM practices to one best model?
-
In a filtration cycle, why does constant-pressure filtration usually occur near the end of the cycle and constant-rate filtration at the beginning?
-
A cyclone of diameter D c of 2 ft, whose dimension ratios are as given in Figure 19.9, is being considered to remove dust from a cement kiln. The gas feed, at inlet velocity i of 20 ft 3 /s, has 0.5...
-
Why have theoretical analyses that treat voids in filter cakes as flow channels not been applied industrially?
-
Janice will need to pay $200 at the end of every month for the next 12 months, except for the payment of the 8th month. What is the present value, assuming a rate of 4%, compounded quarterly?
-
List and explain two management tools in the planning process and two measurable performance indicators. Explain in detail.
-
What does a high PE tell us about the value of the stock price (over or under valued)? What does a low PE tell us about the value of the stock price (over or under valued)? Be specific with your...

Study smarter with the SolutionInn App


