The following table gives the mole fraction of methylbenzene (A) in liquid and gaseous mixtures with butanone
Question:
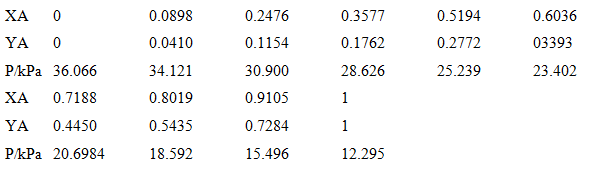
The following table gives the mole fraction of methylbenzene (A) in liquid and gaseous mixtures with butanone at equilibrium at 303.15 K and the total pressure p. Take the vapour to be perfect and calculate the partial pressures of the two components. Plot them against their respective mole fractions in the liquid mixture and find the Henry's law constants for the two components.
Transcribed Image Text:
0.2476 0.3577 0.1762 0.5194 0.6036 0.0898 XA 0.1154 0.0410 0.2772 03393 YA PkPa 36.066 25.239 23.402 28.626 34.121 30.900 0.9105 0.7188 XA 0.8019 0.5435 0.7284 0.4450 YA 12.295 P/kPa 20.6984 15.496 18.592
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
PA YAP and PB BP Daltons law Hence draw up the following table P...View the full answer

Answered By

Shameen Tahir
The following are details of my Areas of Effectiveness. The following are details of my Areas of Effectiveness English Language Proficiency, Organization Behavior , consumer Behavior and Marketing, Communication, Applied Statistics, Research Methods , Cognitive & Affective Processes, Cognitive & Affective Processes, Data Analysis in Research, Human Resources Management ,Research Project,
Social Psychology, Personality Psychology, Introduction to Applied Areas of Psychology,
Behavioral Neurosdence , Historical and Contemporary Issues in Psychology, Measurement in Psychology, experimental Psychology,
Business Ethics Business Ethics An introduction to business studies Organization & Management Legal Environment of Business Information Systems in Organizations Operations Management Global Business Policies Industrial Organization Business Strategy Information Management and Technology Company Structure and Organizational Management Accounting & Auditing Financial Accounting Managerial Accounting Accounting for strategy implementation Financial accounting Introduction to bookkeeping and accounting Marketing Marketing Management Professional Development Strategies Business Communications Business planning Commerce & Technology Human resource management General Management Conflict management Leadership Organizational Leadership Supply Chain Management Law Corporate Strategy Creative Writing Analytical Reading & Writing Other Expertise Risk Management Entrepreneurship Management science Organizational behavior Project management Financial Analysis, Research & Companies Valuation And any kind of Excel Queries.
4.70+
16+ Reviews
34+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physical Chemistry questions
-
The following table gives selective data on nominal exchange rates, price levels, and real exchange rates for Country A and several other countries. Country A uses the dollar (A$) as its currency....
-
The following table gives the total points scored by each of the top 16 National Basketball Association (NBA) scorers during the 201011 regular seasons a. Calculate the mean and median. Do these data...
-
The following table gives the top five television shows, as determined by the Nielsen Ratings for the week ending October 19, 2008. Identify the type of data provided by the information in each...
-
int Temp [10] = { 22, 30, 40,28,20,60}; For the above code, answer the following questions i) Find the size of Temp array. ii) How many memory locations are present in Temp array ? iii) Find the...
-
Explain how the cerebellum is physically connected to the brain stem.
-
Johnson Enterprises uses a computer to handle its sales invoices. Lately, business has been so good that it takes an extra 3 hours per night, plus every third Saturday, to keep up with the volume of...
-
Problem 3.8 asked you to fit two different models to the chemical process data in Table B.5. Perform appropriate residual analyses for both models. Discuss the results of these analyses. Calculate...
-
Smiths Exteriors produces exterior siding for homes. The Preparation Department begins with wood, which is chopped into small bits. At the end of the process, an adhesive is added. Then the...
-
Discuss the concept of sense making and its significance in facilitating sense giving during periods of organizational ambiguity and transformation?
-
Tyler, a single taxpayer, generates business income of $3,000 in 2019. In 2020, he generates an NOL of $5,000. In 2021, he generates business income of $1,000. In 2022, his business generates income...
-
At 18C the total volume V of a solution formed from MgS04 and 1.000 kg of water fits the expression v = 1001.21 + 34.69(x - 0.070)2, where v = V/cm3 and x = blb-1. Calculate the partial molar volumes...
-
Use the Gibbs-Duhem equation to show that the partial molar volume (or any partial molar property) of a component B can be obtained if the partial molar volume (or other property) of A is known for...
-
In Exercises 5160, convert each equation to standard form by completing the square on x and y. Then graph the ellipse and give the location of its foci. 16x 2 + 25y 2 - 300y + 500 = 0
-
Anna is running to the right, as shown in Figure Q3.19. Balls 1 and 2 are thrown toward her by friends standing on the ground. According to Anna, both balls are approaching her at the same speed....
-
A, B, and C are three similar plants under the same management who wants to merge them for better operation. The details are as under: You have to find out: (i) the capacity of the merged plant for...
-
Small Ltd. has been offered a choice to buy one out of two machines, A and B. You are required to compute: (a) Break-even point for each of the machines. (b) The level of sales at which both machines...
-
ABC Company Ltd. expects the following sales by months in units for the first six months of next year. The company has a policy of maintaining an inventory equal to budgeted sales for the following...
-
The Royal Industries Ltd. has prepared its annual sales forecast, expecting to achieve sales of 30,00,000 next year. The controller is uncertain about the pattern of sales to be expected by month and...
-
Bala Varadarajan is one of four cofounders of a skateboard company. The cofounders decided to write a business plan to obtain funding for their venture. During a recent meeting, Bala said, I know...
-
The rate at which the temperature of an object changes is proportional to the difference between its own temperature and the temperature of the surrounding medium. Express this rate as a function of...
-
What are techniques for reducing the risk of drop off, the distortion of a message being sent from top management to subordinates?
-
Calculate the magnitude and direction of the dipole moment of the following arrangement of charges in the xy-plane: 3e at (0,0), e at (0.32 nm, 0), and 2e at an angle of 20 from the x-axis and a...
-
An H 2 O molecule is aligned by an external electric field of strength 1.0 kV m 1 and an Ar atom ( = 1.66 10 24 cm 3 ) is brought up slowly from one side. At what separation is it energetically...
-
The electric dipole moment of toluene (methylbenzene) is 0.4 D. Estimate the dipole moments of the three xylenes (dimethylbenzene). Which answer can you be sure about?
-
The highest WACC is 11%, the baseline WACC is 7.4% and lowest WACCS is 2.8%. To derive a WACC that better accounts for extreme scenarios, you decide to assign the following probability weights: High-...
-
Give 2 examples of the ff: Statutory law Regulatory law Common law Include the title of each example and explain why you chose these examples ( 1 to 2 sentences ) . Note: Write your reference ( s )
-
The Adams family includes a financially well-informed couple, both aged 36, and two children aged 4 and 6. The family is financially sound but suffered badly during the tech meltdown in 2000. The...

Study smarter with the SolutionInn App


