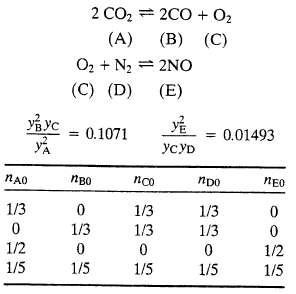
The following two reactions take place in a gas-phase reactor: If the system comes to equilibrium at
Question:
The following two reactions take place in a gas-phase reactor: If the system comes to equilibrium at 3000 K and 1 atm, the product gas mole fractions satisfy the relations
(a) Let nA0 . . . nE0 be the initial number of gram-moles of each species and ?1 and ?2?be the extents of reactions 1 and 2 respectively, at equilibrium (see Equation 4.6-6). Derive expressions for the mole fractions yA, yB . . . ,yE in terms of nA0, nB0 . . . nE0, ?1 and ?2.?Then substitute in the equilibrium relations to derive two simultaneous equations for the two extents of reaction.
(b) One-third of a gram-mole each of CO2, O2, and N2 are charged into a batch reactor and the reactor contents equilibrate at 3000 K and 1 atm. Without doing any calculations, prove that you have enough information to calculate the component mole fractions of the reactor contents at equilibrium.
(c) Perform the calculation of part (b), using either (i) an equation solving program or (ii) a spreadsheet that implements the Newton-Raphson method outlined in Section A.2i of Appendix A. If you use the spreadsheet, guess initial values of 0.1 for both ?1?and ?2 and iterate until successive estimates of these values differ by less than 0.1%.
(d) Write a computer program to implement the Newton?Raphson procedure of part (c) for an arbitrary starting composition. The program should take input values of nA0, nB0, nC0, nD0, and nE0 and calculate the total moles and mole fractions of each species at equilibrium, stopping when the values of and each change by less than 0.001% from one iteration to the next. Run the program for the following feed mixtures:
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





