The reaction A ? B is carried out in a laboratory reactor. According to a published article
Question:
The reaction A ? B is carried out in a laboratory reactor. According to a published article the concentration of A should vary with time as follows: CA = CAO exp (? kt) where CAO is the initial concentration of A in the reactor and k is a constant.
(a) If CA and CAO are in lb-moles/ft3 and t is in minutes, what are the units of k?
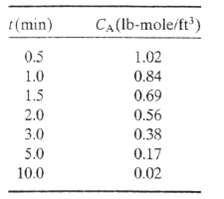
(b) The following data are taken for CA (t); Verify the proposed rate law graphically (first determine what plot should yield a straight line), and calculate CAO and k.
(c) Convert the formula with the calculated constants included to an expression for the morality of A in the reaction mixture in terms of t (seconds). Calculate the morality at t = 200s.?
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





