Ethylbenzene is converted to styrene in the catalytic dehydrogenation reaction C 8 H 10 (g) ? C
Question:
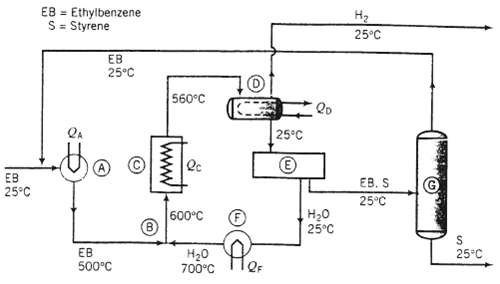
Ethylbenzene is converted to styrene in the catalytic dehydrogenation reaction C8H10 (g) ? C8H8 (g) + H2: ?H?r (600?C) = +124.5 kJ/mol A flowchart of a simplified version of the commercial process is shown here.
Fresh and recycled liquid ethylbenzene combine and are heated from 25?C to 500?C (A), and the heated ethylbenzene is mixed adiabatically with steam at 700?C (B) to produce the feed to the reactor at 600?C. (The steam suppresses undesired side reactions and removes carbon deposited on the catalyst surface.) A once-through conversion of 35% is achieved in the reactor (C), and the products emerge at 560?C. The product stream is cooled to 25?C (D), condensing essentially all of the water, ethylbenzene and styrene and allowing hydrogen to pass out as a recoverable by-product of the process.
The water and hydrocarbon liquids are immiscible and are separated in a settling tank decanter (E). The water is vaporized and heated (F) to produce the steam that mixes with the ethylbenzene feed to the reactor. The hydrocarbon stream leaving the decanter is fed o a distillation tower (G) (actually, a series of towers), which separates the mixture into essentially pure styrene and ethylbenzene each at 25?C after cooling and condensation steps have been carried out. The ethylbenzene is recycled to the reactor pre-heater, and the styrene is taken off as a product.
(a) Calculate on the basis of 100 kg/h styrene produced the required fresh ethylbenzene feed rate, the flow rate of recycled ethylbenzene and the circulation rate of water, all in mol/h. (Assume P = 1 atm.)
(b) Calculate the required rates of heat input or withdrawal in kJ/h for the ethylbenzene pre-heater (A), steam generator (F), and reactor (C).
(c) Suggest possible ways to improve the energy economy of this process.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





