Three hundred L/h of a 20 mole% C 3 H 8 ?80% n-C 4 H 10 gas
Question:
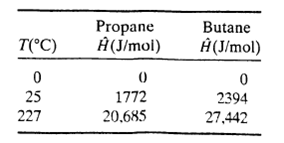
Three hundred L/h of a 20 mole% C3H8?80% n-C4H10 gas mixture at 0oC and 1.1 atm and 200 L/h of a 40 mole% C3H8?60% n-C4H10 mixture at 25?C and 1.1 atm are mixed and heated to 227?C at constant pressure. Calculate the heat requirement in kJ/h. Enthalpies of propane and n-butane are listed below. Assume ideal gas behavior.
Transcribed Image Text:
T(°C) 0 25 227 Propane Ĥ (J/mol) 0 1772 20,685 Butane Ĥ(J/mol) 0 2394 27,442
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
Basis Given feed rates n molh 02 C3Hg 08 C4H10 0C 11 atm Q kJh Mol...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau
Question Posted:
Students also viewed these Chemical Engineering questions
-
Propane gas is burned steadily at 1 atm pressure with a 10 percent excess oxygen supplied by atmospheric air. The reactants enter a steady flow combustor at 25C. Determine the final temperature of...
-
Butane gas at 77 F is mixed with 150% theoretical air at 1000 R and is burned in an adiabatic SSSF combustor. What is the temperature of the products exiting the combustor?
-
In Pissarides's view of labor markets (job matching model), the focus is on finding a good ________. a. level of TFP (A) b. supply of money (M). c. match d. Wage
-
Why is it reasonable to assume that most firms will have a banking relationship?
-
An oil company wants to divest its low-growth chemicals division, which has an estimated stand-alone value of around $5 billion and represents around 40 percent of the entire oil companys value. What...
-
A positively charged particle located at the origin of an \(x y z\) coordinate system spins about the \(z\) axis, and the spin is counterclockwise when viewed looking down from the positive \(z\)...
-
Duff Company is a subsidiary of Rand Corporation and is located in Madrid, Spain, where the currency is the euro (). Data on Duffs inventory and purchases are as follows: Inventory, January 1,...
-
23.Radiation from hydrogen gas excited to first excited state is used for illuminating certain metallic plate. When the same plate is exposed to the radiation from some unknown hydrogen like gas...
-
Which of the graphs in Fig. Q25.12 best illustrates the current I in a real resistor as a function of the potential difference V across it? Explain. Figure Q25.12 (a) (b) (c) (d)
-
The following diagram shows a simplified version of how a refrigerator works: In a liquid receiver (1), a liquid refrigerant (any one of a number of halogenated hydrocarbons such as CC1 2 F 2 ) is...
-
Air at 38C and 97% relative humidity is to be cooled to 18C and fed into a plant area at a race of 510m3/min. (a) Calculate the rate (kg/mm) at which water condenses. (b) Calculate the cooling...
-
Refer to the information in BE65. Calculate ending inventory and cost of goods sold for 2015, assuming the company uses specific identification. Actual sales by the company include its entire...
-
The answer of the fiurth line is not 4, 8, 32 or 40. I just need the answer for the fourth line Question 1 Memory Allocation SUBMITTED How much memory (in bytes) is taken up by the following...
-
Goga is planning his day off. He wants to get up late, swim, and watch TV . In addition, he needs to earn some money by collecting leaves in the neighbors' yards. Goga knows 7 neighbors who want to...
-
With Distributed event matching (DEM) the following discussions are centered on it. 1.Discuss cases of digital transparency DEM may provide relating to implementation styles. 2.Discuss the cases of...
-
Valuation for ZipCo Limited (4 marks): Apply three valuation models, i.e. residual income model, residual operating income model and free cash flow model, to estimate the intrinsic value of the...
-
Two stars are orbiting each other in a binary system. If one star has a mass of 4.0 10 30 kg, the other star has a mass of 2.0 10 30 kg, and if they are 1.7 10 14 m apart, what is the force of...
-
Redo question 42 using the data for Ford. Question 42 Estimate the regression of Chryslers return on assets against its debt/equity ratio. Compute the DurbinWatson statistic. Does autocorrelation...
-
Discuss the information available from the following techniques in the analysis of inorganic pigments used in antique oil paintings: (i) Powder X-ray diffraction, (ii) Infrared and Raman...
-
Solve the differential equation. y" + 4y' + 4y = 0
-
Calculate the molar H3O+ ion concentration of a solution that has a pH of (a) 4. 31. (b) 0.59. (c) 7.62. (d) 20.76.
-
Calculate the p-functions for each ion in a solution that is (a) 0.0300 M in NaBr. (b) 5.5 3 10-3 M in Ba(OH)2. (c) 8.7 3 10-3 M in CaCl2 and 6.6 x 10-3 M in BaCl2
-
Convert the following p-functions to molar concentrations: (a) pH = 1.020. (b) pBr = 7.77. (c) pLi = 12.35. (d) pMn = 0.135.
-
Discuss feminist ideology a) What is feminism as an ideology? How do feminists explain and understand the root cause of gender inequality and what do they propose to do? Include a discussion of...
-
You invest $ 1 , 1 0 0 at a 1 2 % annual interest rate, stated as an APR. Interest is compounded monthly. How much will you have in 1 . 5 years? In 2 years? ( Do not round intermediate calculations....
-
"Beginning Transactions" on the first Excel worksheet will provide the Balance Sheet for 2019 and the Transactions for January 2020. On the second Excel worksheet, "Journal Entries" , you should...

Study smarter with the SolutionInn App


