Using the SRK equation of state (or any other cubic equation of state) to determine a specific
Question:
Using the SRK equation of state (or any other cubic equation of state) to determine a specific volume from a specified temperature and pressure requires a trial-and-error calculation. Three computer- based approaches to solving this problem may be used: (1) spread sheeting; (2) mathematical packages such as MathCAD, Mathematica, Maple, and E-Z Solve: and (3) programming languages such as Fortran and C++, the goal of this problem is to use each approach to determine V (L/mol) for CO, at (i) 200 K and 6.8 atm; (ii) 250 K and 12.3 atm; (iii) 300 K and 6.8 atm; (iv) 300 K and 21.5 atm; and (v) 300 K and 50.0 atm.
(a) Starting with Equation 5.3-7, derive the following equivalent expression for the SRK equation of State: f(V) = PV3 ? RTV2 + (aa ? b2P ? bRT)V ? aab = 0
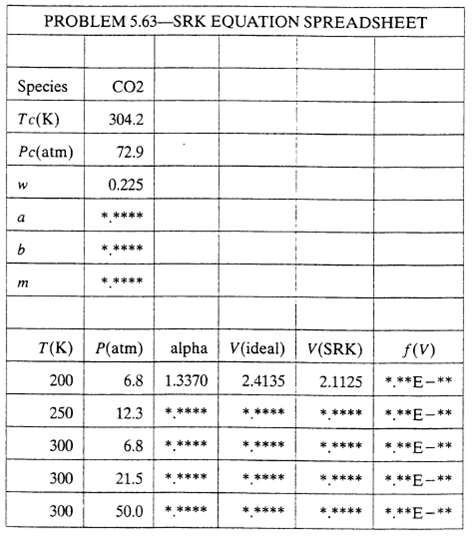
(b) Write a spreadsheet program to take as inputs a species identifier (such as C02), the critical temperature, critical pressure and Pitzer a centric factor, and the temperatures and pressures for which V is to be calculated, and to calculate V using Equations 5.3-9 to 5.3-13 for each of the specified conditions. The spreadsheet should have the following structure: Single digits should appear in place of each asterisk shown on the table. Formulas should be entered into the row for 200 K and 6.8 atm and copied into the next four rows. The goal seek tool should be used to determine each V(SRK), starting with the ideal gas value and varying the cell value to make f(V) as close as possible to zero.
(c) Use a root-finding procedure in a mathematical software package to determine V for each of the five conditions.
(d) Write a program (in FORTRAN or another programming language) to determine v for each of the five conditions using Newton?s rule (Appendix A.2d). The program should
(I) Read in values of the species formula (CO2), the critical temperature and pressure and the Pitzer a centric factor.
(ii) Calculate a, b, and m.
(iii) Read values of T and P for which V is to be calculated. Terminate if a negative value of T is input.
(iv) Use the ideal gas equation of state to generate the initial value of V
(v) Calculate and print successive estimates of V using Equation A.2-2 stopping when the fractional change in V from one iteration to the next (? of Equation A.2-8) is less than 1.0 x 10-5. Build in an upper limit of 15 iterations for each process condition: if convergence is not achieved within that limit, print an error message and quit.
(vi) Go back to Step (iii)
(e) Briefly summarize the advantages and disadvantages of the three problem-solving approaches.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





