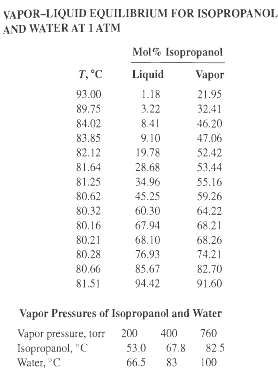
Vapor-liquid equilibrium data for mixtures of water and isopropanol at 1 atm (101.3 kPa, 760 torr) are
Question:
Vapor-liquid equilibrium data for mixtures of water and isopropanol at 1 atm (101.3 kPa, 760 torr) are given below.
(a) Prepare T-x-y and x-y diagrams.
(b) When a solution containing 40 mol % isopropanol is slowly vaporized, what will be the composition of the initial vapor formed?
(c) If this same 4.0% mixture is heated under equilibrium conditions until 75 mol% has been vaporized, what will be the compositions of the vapor and liquid produced?
(d) Calculate K-values and values of a at 80?C and 89?C.
(e) Compare your answers in parts (a), (b), and (c) to those obtained from T-x-y and x-y diagrams based on the following vapor pressure data and Raoult's and Dalton's laws. What do you conclude about the applicability of Raoult's law to thissystem?
Step by Step Answer:






