(a) Find the bubble-point temperature of the following mixture at 50 psia, using K-values from figure. (b)...
Question:
(a) Find the bubble-point temperature of the following mixture at 50 psia, using K-values from figure.
(b) Find the temperature that results in 25% vaporization at this pressure. Determine the corresponding liquid and vaporcompositions.
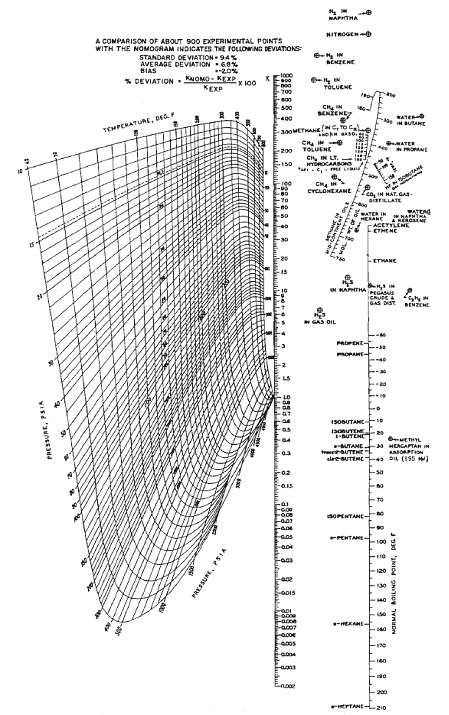
Transcribed Image Text:
NAPHTHA NITROGEN A COMPARISON OF ABOUT 900 EXPERIMENTAL POINTS WITH THE NOMOGRAM INDICAT ES THE FOLLOWNG DEVIATIONS STANDARD OEVIATION- 94% AVERAGE DEVATION 6.% BENZEHE BIAS -1000 * DEVIATION . KNOMO- KEXP X 100 TOLUENE KEXP -700 -800 CH N SENŽENEY 500 RATE -400 TEMPERATURc, eF -300METHANENG, TOC ATER TOLUENE 200 CH, IN LT. mROCAABOa -ree CYCLONEXANE Lea.IN HAT.GA கர்டடAா MATER IN HERANE GME ACEYYLENE CTHENC *RAPH ETHANE MATHAA IN BENZEHE GAS DIL PROPENE PROPANE- 1SOBUTANE IaonuTEE -BUTENE UTANE teaUTEHt eleesUTEME DL (155 20 - HEACAPTAN IN AASORPTION -02 -sa -0.15 -ai Fo.ce sOPENTANE- PEHTANE- ఉ చింి 70,00- T-acce -a007 HEKAME- 140 200 EPTANEL210 wietui.LILJ PRKSSURE. PSIA
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
Instead of using Figs 28 and 29 for Kvalues use SRK method with the CHEMCAD simulator with the follo...View the full answer

Answered By

Sheikh Muhammad Ibrahim
During the course of my study, I have worked as a private tutor. I have taught Maths and Physics to O'Level and A'Level students, as well as I have also taught basic engineering courses to my juniors in the university. Engineering intrigues me alot because it a world full of ideas. I have passionately taught students and this made me learn alot. Teaching algebra and basic calculus, from the very basics of it made me very patient. Therefore, I know many tricks to make your work easier for you. I believe that every student has a potential to work himself. I am just here to polish your skills. I am a bright student in my university. My juniors are always happy from me because I help in their assignments and they are never late.
4.90+
14+ Reviews
24+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
The state of liquid water is changed from 50 psia and 50oF to 2000 psia and 100oF. Determine the change in the internal energy and enthalpy of water on the basis of the (a) Compressed liquid tables,...
-
A hydrocarbon vapor-liquid mixture at 250?F and 500 psia contains N2, H2S, CO2, and all the normal paraffins from methane to heptane. Use Figure to estimate the K-value of each component in the...
-
A saturated liquid feed at 125 psia contains 200 lbmol/h of 5 mol% iC4, 20 mol% nC4, 35 mol% iC5, and 40 mol% nC5. This feed is to be distilled at 125 psia with a column equipped with a total...
-
Histogram. Suppose that the standard input stream is a sequence of double values. Write a program that takes an integer n and two real numbers lo and hi as command-line arguments and uses StdDraw to...
-
Prepare the current assets section of the statement of financial position for Nias Corporation, which reported the following selected items at February 28, 2012: Accounts payable Accounts receivable...
-
The Thompson Corporation, a manufacturer of steel products, began operations on October 1, 2016. The accounting department of Thompson has started the fixed-asset and depreciation schedule presented...
-
A tension test was performed on a steel specimen having an original diameter of 12.5mm and gage length of 50mm. Using the data listed in the table, plot the stress-strain diagram, and determine...
-
Beale Management has a noncontributory, defined benefit pension plan. On December 31, 2011 (the end of Beale's fiscal year), the following pension-related data were available: Required: 1. Prepare...
-
A1500 kg aircraft going35 m/s collides with a2000 kg aircraft that is parked and they stick together after the collision and are going 15 m/s after the collision. If they skid for 106.8 m before...
-
A company has two factories, one at Liverpool and one at Brighton.[20] In addition it has four depots with storage facilities at Newcastle, Birmingham, London and Exeter. The company sells its...
-
In Figure, 150 kmol/h of a saturated liquid, L1, at 758kPa, of molar composition, propane lo%, n-butane 40%, and n-pentane 50%, enters the reboiler from stage 1. What are the compositions and amounts...
-
As shown in Figure, a hydrocarbon mixture is heated and expanded before entering a distillation column. Calculate, using a simulation computer program, the mole percent vapor phase and vapor and...
-
Describe automated matching in the purchasing process and explain how this IT enablement has improved efficiency in companies.
-
Terry bought a house for 65,000 on 1 June 1997 and occupied the house as his principal private residence. He lived in the house until 1 June 2003 when he went to stay with relatives in Australia,...
-
Jeremy acquired the following ordinary shares in Scarlon plc: Date 19 September 2009 20 October 2011 21 November 2013 22 December 2017 13 January 2018 No of shares 4,000 2,000 1,000 3,000 5,000 Cost...
-
In May 2012, Ruth sold a freehold building which she had used exclusively for business purposes. The building was sold for 220,000, realising a chargeable gain of 42,500. In the following month, Ruth...
-
Sandra acquired the following ordinary shares in Pincom plc: Date 29 January 1995 13 August 1999 4 October 2011 No of shares (a) 8,400 (b) 6,300 (c) 5,200. 1,000 1,000 2,000 Cost 4,000 9,500 22,500...
-
Rupert bought a house in Manchester on 1 November 1995 for 75,000. He occupied the house until 1 November 1999 when he left to work abroad for a year, moving back into the house on 1 November 2000....
-
Explain the significance of a party transferring a negotiable instrument endorsed without recourse. When is this type of endorsement used?
-
Distinguish between the work performed by public accountants and the work performed by accountants in commerce and industry and in not-for-profit organisations.
-
Explain why an alpha measure based on the domestic CAPM model is inappropriate for assessing the stock selection ability of a global mutual fund manager. Indicate in which situations an alpha measure...
-
A mixture of 16,500 kg/h of 55 wt% methyl acetate and 45 wt% methanol is to be separated into 99.5 wt% methyl acetate and 99 wt% methanol. Use of one homogeneous azeotropic distillation column and...
-
Why is CO2 frequently a desirable solvent for SFE?
-
What happens to the solvent power of a compressed gas as it passes through the critical region? What happens to physical properties in the critical region?
-
Mrs. Sam Taffer was a brilliant engineer at IBM and made a lot of money from stock options. Since then, she has worked as a musician and raised her two sons. She wishes to have most of her property...
-
Miller Company ended its fiscal year on June 30, 2017. The company's adjusted trial balance as of the end of its fiscal year is shown below. MILLER COMPANY Adjusted Trial Balance June 30, 2017...
-
What depreciation method is used to write off property, plant and equipment? Regarding the triple bottom line, what information did the company disclose about environmental matters in the reports?...

Study smarter with the SolutionInn App


