An aqueous glycerol solution weighing 100.0 mg was treated with 50.0 mL of 0.083 7 M Ce
Question:
An aqueous glycerol solution weighing 100.0 mg was treated with 50.0 mL of 0.083 7 M Ce4+ in 4 M HClO4 at 60°C for 15 min to oxidize the glycerol to formic acid:
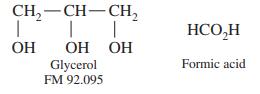
The excess Ce4+ required 12.11 mL of 0.044 8 M Fe2+ to reach a ferroin end point. What is the weight percent of glycerol in the unknown?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





