An SO2-air mixture is being scrubbed with water in a countercurrent-flow packed tower operating at 20C and
Question:
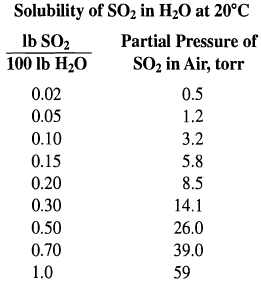
An SO2-air mixture is being scrubbed with water in a countercurrent-flow packed tower operating at 20°C and 1 atm. Solute-free water enters the top of the tower at a constant rate of 1,000 lb/h and is well distributed over the packing. The liquor leaving contains 0.6 lb S02/100 lb of solute-free water. The partial pressure of SO2 in the spent gas leaving the top of the tower is 23 ton. The mole ratio of water to air is 25. The necessary - equilibrium data are given below.
(a) What percent of the SO2 in the entering gases is absorbed in the tower?
(b) In operating the tower it was found that the rate coefficients kp and kL remained substantially constant throughout the tower at the following values:

At a point in the tower where the liquid concentration is 0.001 lbmol SO2 per lbmol of water, what is the liquid concentration at the gas-liquid interface in lbmol/ft3? Assume that the solution has the same density asH20.
Step by Step Answer:






