Figure shows two paths that may be taken by a gas from an initial point i to
Question:
Figure shows two paths that may be taken by a gas from an initial point i to a final point f. Path 1 consists of an isothermal expansion (work is 50 J in magnitude), an adiabatic expansion (work is 40 J in magnitude), an isothermal compression (work is 30 J in magnitude), and then an adiabatic compression (work is 25 J in magnitude). What is the change in the internal energy of the gas if the gas goes from point I to point f along path2?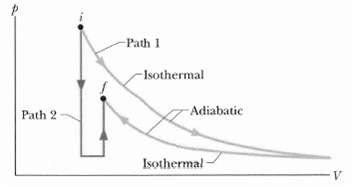
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals of Physics
ISBN: 978-0471758013
8th Extended edition
Authors: Jearl Walker, Halliday Resnick
Question Posted:





