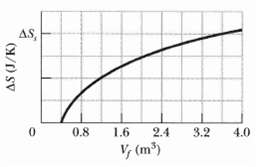
A gas sample undergoes a reversible isothermal expansion. Figure gives the change S in entropy of the
Question:
A gas sample undergoes a reversible isothermal expansion. Figure gives the change ΔS in entropy of the gas versus the final volume Vf of the gas. The scale of the vertical axis is set by ΔSs = 64 J/K. How many moles are in the sample?
Transcribed Image Text:
AS, 0.8 1.6 2.4 3.2 4.0 V, (m)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
We concentrate on the first term of Eq 204 the second term is zero because the f...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals of Physics
ISBN: 978-0471758013
8th Extended edition
Authors: Jearl Walker, Halliday Resnick
Question Posted:
Students also viewed these Thermodynamics questions
-
How many moles are present in 3.55 1024 Pb atoms?
-
How many moles are present in 2.09 1022 Ti atoms?
-
How many moles are present in 1.00 1023 PF3 molecules?
-
What are the main advantages and disadvantages of using 360 degree appraisal?
-
What is meant by the optimum tariff? What is its relationship to changes in the nation's terms of trade and volume of trade?
-
A student performed an experiment with three different grips to see what effect it might have on the distance of a backhanded Frisbee throw. She tried it with her normal grip, with one finger out,...
-
Give two reasons to explain why a company usually seeks to manage rather than eliminate its risks.
-
The Lawson Manufacturing Company has the following budgeted overhead cost and other data for its assembly department for the month of April: Budgeted Data: Indirect labor and supplies . $170,000...
-
1.A transformer has 210turns in the primary coil and 30 turns in the secondary coil. 2.When a 20 V potential difference and 800 W power are outputted from the secondary coil, 3.what is the potential...
-
Using scapy to hijack a telnet session and insert your own commands. If easier can provide your program with the packet sequence number. Metasploit VM has a Telnet server that can be attacked. Submit...
-
In the irreversible process of Figure let the initial temperatures of identical blocks L and R be 305.5 and 294.5 K, respectively, and let 215 J be the energy that must be transferred between the...
-
A 50.0 g block of copper whose temperature is 400 K is placed in an insulating box with a 100 g block of lead whose temperature is 200 K. (a) What is the equilibrium temperature of the two-block...
-
Evaluate the integral. tan 6 (ay) dy
-
How does ethnocentrism manifest in the context of cultural anthropology and sociology, particularly concerning the evaluation of other societies based on one's own cultural standards?
-
What is the purpose of the revaluation reserve account? When should the account be debited and when will it be credited?
-
Theo's Tree Trimming Service reported total revenue of $100,000 with explicit costs of $20,000. The opportunity cost of labor (an implicit cost) is $15,000. What is Theo's economic profit?
-
At what point would the IRS typically start paying interest on a refund due to a taxpayer who electronically filed their return before the due date?
-
What is a Revenue Ruling, and its main purpose? At the top of the Rev. Rul. There is one code section. What is it ? Properly format your citations. What is a Private Letter Ruling, and how does...
-
Define the covariance and correlation between two random variables, and compute these values given a joint probability function of two discrete random variables.
-
Differentiate the following terms/concepts: a. Personality types and money attitudes b. Planners and avoiders c. Moderating and adapting to biases d. "Perfectible judges" and "incorrigible judges"
-
Two football players collide head-on in midair while trying to catch a thrown football. The first player is 95.0 kg and has an initial velocity of 6.00 m/s, while the second player is 115 kg and has...
-
Two loudspeakers, A and B (Fig. 16.40), are driven by the same amplifier and emit sinusoidal waves in phase. Speaker B is 2.00 m to the right of speaker A. Consider point Q along the extension of the...
-
Two loudspeakers, A and B (Fig. 16.40), are driven by the same amplifier and emit sinusoidal waves in phase. Speaker B is 2.00 m to the right of speaker A. The frequency of the sound waves produced...
-
Two loudspeakers, A and B, are driven by the same amplifier and emit sinusoidal waves in phase. Speaker B is 12.0 m to the right of speaker A. The frequency of the waves emitted by each speaker is...
-
Assume that in 2023, Bobby had a vacant lot that was given to him as a gift from his father that had an FMV of $40,000 and an adjusted basis of $15,000.
-
Show the adjusting entries that you would make given the additional data below. In addition to the formal journal entry, please also indicate the type of adjusting entry that is being made, ...
-
I need one to three paragraphs written about the awk command in unix. Everything should be in a power point presentation. Make sure it contains the following: Command name Description How to use the...

Study smarter with the SolutionInn App


