Solutions with a wide range of Hg 2+ concentrations were prepared to calibrate an experimental Hg 2+
Question:
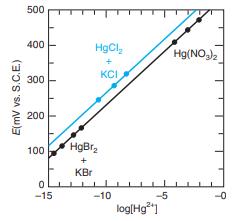
Solutions with a wide range of Hg2+ concentrations were prepared to calibrate an experimental Hg2+ ion-selective electrode. For the range 10 -5 < [Hg2+] [< 10-1 M, Hg(NO3)2 was used directly. The range 10-11 <[Hg2+] < 10-6 M could be covered by the buffer system HgCl2(s) + KCl(aq) (based on pKsp for HgCl2 = 13.16). The range 10-15 < [Hg2+] < 10-11 M was obtained with HgBr2(s) + KBr(aq) (based on pKsp for HgBr2 = 17.43). The resulting calibration curve is shown in the figure. Calibration points for the HgCl2/ KCl buffer are not in line with the other data. Suggest a possible explanation.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





