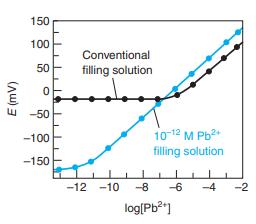
The Pb 2+ ion buffer used inside the electrode for the colored curve in Figure 14-27 was
Question:
The Pb2+ ion buffer used inside the electrode for the colored curve in Figure 14-27 was prepared by mixing 1.0 mL of 0.10 M Pb(NO3)2 with 100.0 mL of 0.050 M Na2EDTA. At the measured pH of 4.34,α γ4- = 1.46 × 10-8 (Equation 11-4). Show that [Pb2+] = 1.4 × 10-12 M.
Figure 14-27
Equation 11-4
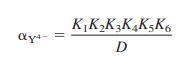
Transcribed Image Text:
150 100 Conventional 50 filling solution -50 10-12 M Pb2 filling solution -100 -150 -12 -10 -8 -6 -4 -2 log[Pb?"] (Aw) 3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
There is a large excess of EDTA in the buffer We expect essentially ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
Solutions with a wide range of Hg 2+ concentrations were prepared to calibrate an experimental Hg 2+ ion-selective electrode. For the range 10 -5 < [Hg 2+ ] [ < 10 -1 M, Hg(NO 3 ) 2 was used...
-
(a) Which ion is smaller, Co3+ or Co4+? (b) In a lithium ion battery that is discharging to power a device, for every Li+ that inserts into the lithium cobalt oxide electrode, a Co4+ ion must be...
-
Suppose that the Ag | AgCl outer electrode in Figure 14-11 is filled with 0.1 M NaCl instead of saturated KCl. Suppose that the electrode is calibrated in a dilute buffer containing 0.1 M KCl at pH...
-
Given a sorted array of Comparable items, write functions floor () and ceiling () that return the index of the largest (or smallest) item not larger (or smaller) than an argument item in logarithmic...
-
Betancourt Company sells automatic can openers under a 75-day warranty for defective merchandise. Based on past experience, Betancourt estimates that 3% of the units sold will become defective during...
-
The block diagram of Fig. 1.b represents the heading control of the traditional bi-wing aircraft in Fig. 1.a. Aa Controller Engine dysunkc 100 10 Design a control system for the bi-wing aircraft to...
-
Give an explicit solution for the mean-reverting rnstein-Uhlenbeck SDE \[d X_{t}=\alpha\left(\mu-X_{t} ight) d t+\sigma d B_{t}\] with \(X_{0}=x\).
-
Irene contributes land to Micro Development Partnership for a 30% interest. The land's basis is $20,000, and it has a fair market value of $80,000. Micro reports a net operating loss of $100,000 for...
-
what are the efficacious strategies for orchestrating structural transformations within complex organizational ecosystems, ensuring congruence between strategic imperatives, organizational...
-
Consider the following transactions for Burlington Drug Store: Feb. 2 Burlington buys $23,800 worth of inventory on account with credit terms of 2/15, n/30, FOB shipping point. 4 Burlington pays a...
-
A Ca 2+ ion-selective electrode was calibrated in metal ion buffers with ionic strength fixed at 0.50 M. Using the following electrode readings, write an equation for the response of the electrode to...
-
Activity problem. Citric acid is a triprotic acid (H 3 A) whose anion (A 3- ) forms stable complexes with many metal ions. When a Ca 2+ ion-selective electrode with a slope of 29.58 mV was immersed...
-
To finance its working capital needs, Omega company needs to borrow $600,000 for a period of 6 months. For this purpose, Omega is considering three options: a. A loan from a microfinance organization...
-
Huang Company presented the following data (yen in thousands). Instructions Compute earnings per share. Net income Preference shares: 50,000 shares outstanding, 100 par, 8% cumulative, not...
-
Consider a sample taken from the population of all taxi-in times for all flights that land in Los Angeles. Identify the symbols used for the sample mean and the population mean.
-
A portion of the statement of income and retained earnings of Pierson Inc. for the current year follows. During the year, Pierson Inc. had a loss from discontinued operations of \($1\),340,000 after...
-
At January 1, 2015, Cameron Companys outstanding shares included the following. Net income for 2015 was R\($2\),830,000. No cash dividends were declared or paid during 2015. On February 15, 2016,...
-
Amy Dyken, controller at Fitzgerald Pharmaceutical Industries, a public company, is currently preparing the calculation for basic and diluted earnings per share and the related disclosure for...
-
How can constructive and destructive interference be reconciled with the principle of conservation of energy?
-
Copy and complete the statement. 3800 m ? km =
-
A 4.36-g sample of an unknown alkali metal hydroxide is dissolved in 100.0 mL of water. An acid-base indicator is added and the resulting solution is titrated with 2.50 M HCl(aq) solution. The...
-
An 8.65-g sample of an unknown group 2A metal hydroxide is dissolved in 85.0 mL of water. An acid-base indicator is added and the resulting solution is titrated with 2.50 M HCl(aq) solution. The...
-
A solution of 100.0 mL of 0.200 M KOH is mixed with a solution of 200.0 mL of 0.150 M NiSO4. (a) Write the balanced chemical equation for the reaction that occurs. (b) What precipitate forms? (c)...
-
Popular furniture company, IKEA, has purchased forests in Romania as well as land in Alabama to assist with keeping up with the wood demand necessary to complete customer orders. This was one way...
-
How does China being Turkey's biggest import partner affect Turkey's exchange rate?
-
Assignment 4 In this assignment you are provided information on an experiment and you are required to investigate and interpret the output which is provided below. Problem: Consider the...

Study smarter with the SolutionInn App


