A chemical process recovers heat by transferring energy from a gas stream to a cold brine stream.
Question:
A chemical process recovers heat by transferring energy from a gas stream to a cold brine stream. During the heating of the brine, salts deposit on the heat-exchanger surfaces and cause significant fouling. The consequence of this fouling is that the heat transferred to the brine is reduced, causing additional heat, in the form of condensing steam, to be required later on in the process. A portion of a process flow diagram is shown in Figure P14.10.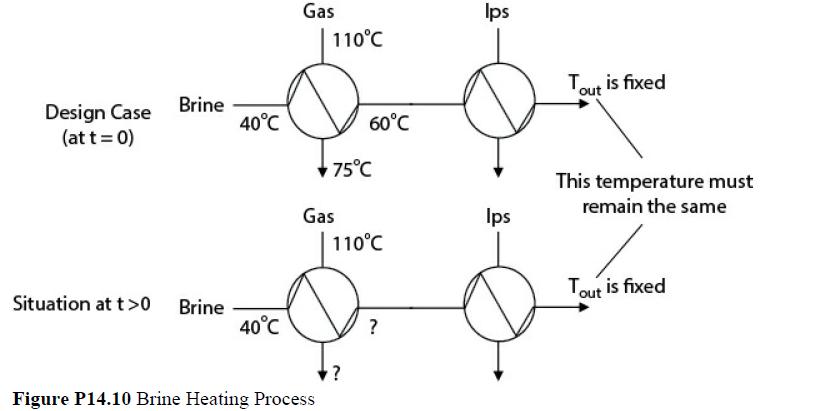
It is known that the heat transfer coefficient for a brine/gas heat exchanger changes as the following function of time:![]()
where t is the time in months after cleaning the heat exchanger, and U is the overall heat transfer coefficient.
The cost of cleaning the exchanger is $15,000, but there is no downtime for the process. At design conditions (unfouled exchanger surface), the flow of gas (cp = 800 J/kg°C) is 40,000 kg/h, and the flow of brine (cp = 4000 J/kg°C) is 14,000 kg/h. The gas enters the heat exchanger at a temperature of 110°C and leaves at 75°C. The brine enters the exchanger at 40°C and leaves at 60°C. The exchanger has an LMTD correction factor F = 0.9, which may be assumed not to change during the fouling process. If the flows and inlet temperatures of the streams remain constant, what is the optimal cycle time for cleaning the heat exchanger? Assume that as the brine outlet temperature drops from 60°C due to the fouling of the exchanger surface, additional low-pressure steam at a cost of $8.00/GJ is used to heat the brine back to 60°C.
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





