a. Derive an equation for the ratio (eta=) rate of cumene formation/rate of DIPB formation Rewrite the
Question:
a. Derive an equation for the ratio \(\eta=\) rate of cumene formation/rate of DIPB formation Rewrite the equation in the form \(\eta=A / B-1\).
b. Present the equation for \(\eta\) given above in terms of the following variables: \(T, P, k_{o}, E_{a c t}\), and mole fractions.
c. Using the equation from Part (b), determine the trend resulting from a change in the following variables. After each item, provide \(\uparrow\) (increases), \(\downarrow\) (decreases), \(\varnothing\) (constant) or ? (cannot be determined).
(i) Increasing system temperature
(ii) Increasing system pressure
(iii) Increasing benzene mole fraction
(iv) Increasing propylene mole fraction
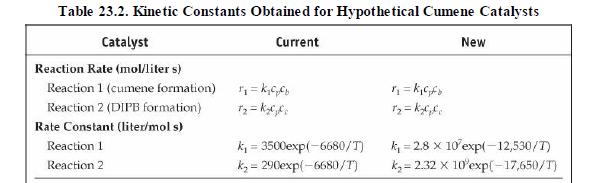
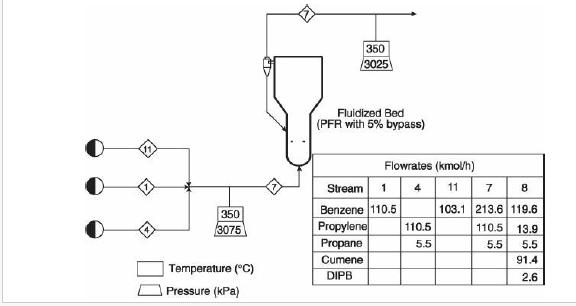
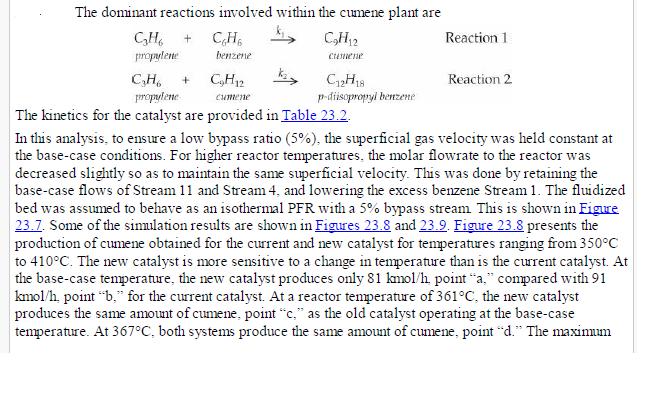
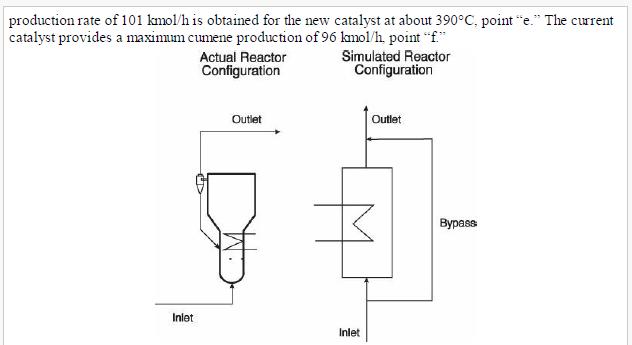
(v) Increasing propane mole fraction (vi) Increasing cumene mole fraction (vii) Increasing DIPB mole fraction Problems 23. 17-23.20 investigate the performance of the cumene reactor (fluidized bed), described in Section 23. 5.2, that results from replacing the catalyst. The replacement catalyst kinetics are given in Table 23. 2. The base case selected is the currently operating reactor (with an active volume of \(\left.7.49 \mathrm{~m}^{3}ight)\). Process output for the base case is given in the flow table in Figure 23. 6.
Figure 23.6
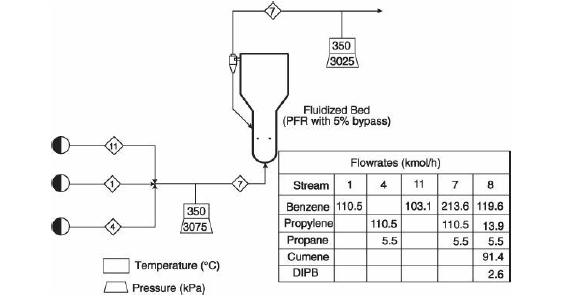
Table 23.2
Data from section 23.52


Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780132618120
4th Edition
Authors: Richard Turton, Richard C. Bailie, Wallace B. Whiting, Joseph A. Shaeiwitz, Debangsu Bhattacharyya





