A liquid-phase biological reaction is used to produce an intermediate chemical for use in the pharmaceutical industry.
Question:
A liquid-phase biological reaction is used to produce an intermediate chemical for use in the pharmaceutical industry. The reaction occurs in a large, well-stirred bioreactor that is illustrated in Figure P14.8. Because this chemical is temperature sensitive, the maximum operating temperature in the reactor is limited to 65°C. The feed material is fed to the reactor through a heat exchanger that can increase the temperature of the reactants (contents of the reactor), which in turn increases the rate of the reaction. This is illustrated in Figure P14.8. The time spent in the bioreactor (space time) must be adjusted in order to obtain the desired conversion of reactant. As the temperature in the reactor increases, so does the reaction rate, and hence the size (and cost) of the reactor required to give the desired conversion decreases. It is desired to determine the optimal temperature at which to maintain the reactor (and to preheat the feed). The costs to be considered are the purchase cost of the reactor and heat exchanger and the operating cost for the energy to heat the feed.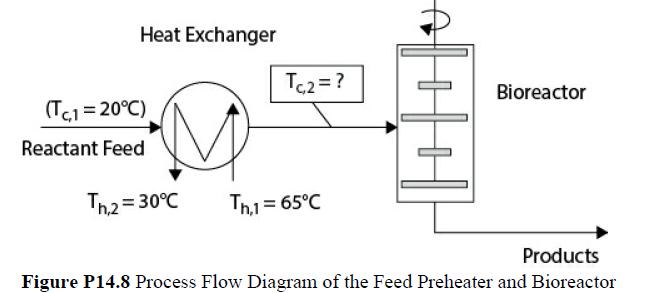
It is desired to optimize the preheat temperature for a flow of 5000 gal/h. The feed has the properties of water (ρ = 1000 kg/m3, Cp = 4.18 kJ/kg°C) and enters the heat exchanger at a temperature of 20°C. Reactor feed is to be heated with a heating medium that is available at a temperature of 65°C and must leave the heat exchanger at 30°C. Therefore, the desired reactor inlet temperature is adjusted by changing the flowrate of the heating medium. The physical properties of the heating medium are ρ = 920 kg/m3, and Cp = 2.2 kJ/kg°C.
The reaction rate for this reaction, −rA, is given in terms of the concentration of reactant A (CA) by the following equation:![where -TA = KCA 3300 k[s=] = 7 exp{-} (P14.8.1) (P14.8.2)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/3/4/9/883654a057b5f8821699349880629.jpg)
The design equation for the reactor is given by
where V is the reactor volume (m3), vo is the volumetric flowrate of fluid into the reactor (m3/s), and XA is the conversion (assumed to be 80%, or 0.8, for this reaction).
The design equation for the heat exchanger is given by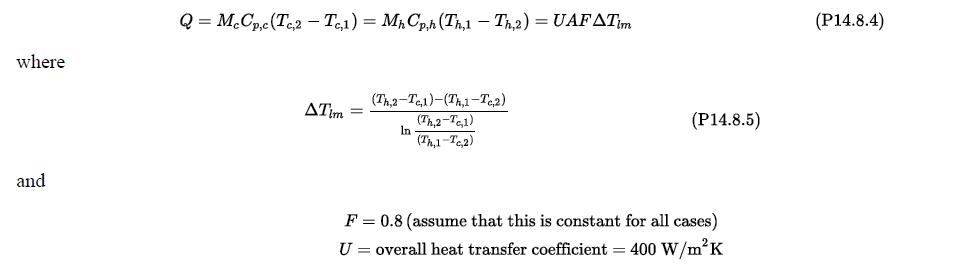
The optimal reactor inlet temperature is the one that minimizes the equivalent annual operating costs (EAOC) given by![2 EAOC[$/y] = PC; [$](A/P, i, n)[/y] + UC[$/y] i=1 (P14.8.6)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/3/4/9/954654a05c2c8feb1699349951779.jpg)
where PCi is the purchase equipment cost for the heat exchanger and reactor and UC is the operating (utility) cost for the heating medium. For this problem use i = 7% p.a. and n = 12 years.
The purchase cost of the reactor is given by![]()
where V is the volume of the reactor in m3. The cost of the heat exchanger is![]()
where A is the area of the heat exchanger in m3. The cost of the heating medium is![]()
Present your final results as two plots. The first should show how each term in Equation (P14.8.6) changes with TC,2,and the second plot should show the EAOC (y-axis) as a function of TC,2,(x-axis). Explain the reason for the trends seen in each of these plots.
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting




