Consider the production of cumene (c) from the catalytic alkylation reaction of benzene (b) using propylene (p)
Question:
Consider the production of cumene (c) from the catalytic alkylation reaction of benzene (b) using propylene (p)— Reaction 1. A second undesirable reaction (Reaction 2) between propylene and cumene occurs to produce pdiisopropyl benzene (DIPB):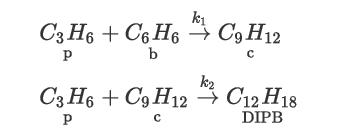
where r1 = k1 cp cb and r2 = k2 cp cb and the rates of reaction are given in mol/L/s and the rate constants are![k = 2.8 x 107 exp = 2.32 x 10 exp k2= 12,530 T[K] and 17,650 170). These reactions are quite](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/4/4/1/388654b6aecae9931699441387138.jpg)
exothermic, and typical reactor temperatures are in the range 350°C to 410°C. The heats of reaction per mol of product in this temperature range are −100.6 kJ/mol and 121.3 kJ/mol for Reactions 1 and 2, respectively. The heat capacities for the reactant and product streams may be taken to be the same and equal to 2.44 kJ/kg/K in this range. For this system, do the following:
1. Determine the conversion of an equimolar feed of propylene and benzene (feed conditions are 350°C, 3 MPa, and 100 mol/s) if the reactor is run as an adiabatic packed bed with a maximum outlet temperature of 425°C.
2. Based on the results from Part (a), is it feasible to obtain an overall conversion of 80% of the benzene using a series of staged packed beds with cooling between stages to bring the temperature back to 350°C at the inlet of the next reactor stage? In practice, no more than four stages would be used.
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





