Consider the reaction in Example 22.4, which described the isothermal liquid-phase conversion of feed A to product
Question:
Consider the reaction in Example 22.4, which described the isothermal liquid-phase conversion of feed A to product B in a plug flow reactor. The reactor volume was found for a given temperature, and a conversion of 95% to be 304 L. The following data were provided:![]()
CAo = 10 mol/L, the feed to the isothermal reactor was FAo = 80 mol/s, and the design equation (Equation [22.32]) was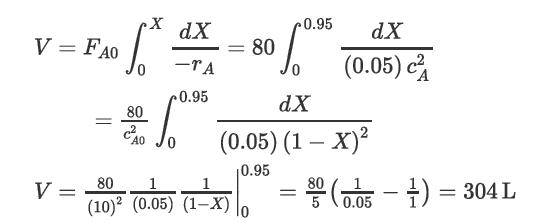
Now consider the case when the reactor is up and running, but the throughput to the reactor is increased by 20%. It may be assumed that heat generation within the reactor is negligible, that is, the heat of reaction is small. For this reactor, consider the following:
1. What will the new conversion be (assuming the temperature remains constant)?
2. If the original temperature of the reactor was 150°C, what should the temperature in the reactor be adjusted to so that the same conversion is achieved with the new throughput? The activation energy for this reaction is E = 20 kJ/mol.
Example 22.4
Consider a simple liquid phase reaction in which reactant A is converted to product B. The reaction is second order in A and the kinetics are given by the equation![-TA (reaction rate) = mol/L/s = 0.05c [mol/L]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/4/3/9/289654b62b9c1dc51699439286963.jpg)
A stream of material A of concentration 10 mol/L is fed to an isothermal reactor at a rate of 8 L/s. Determine the volume of the reactor to obtain 95% conversion of A assuming 1. A CSTR is used 2. A PFR is used
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





