Using the data from Example 22.3, determine the volume of catalyst needed to convert 80% of a
Question:
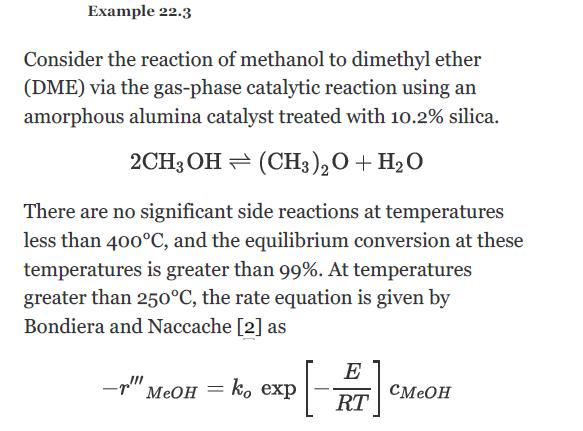
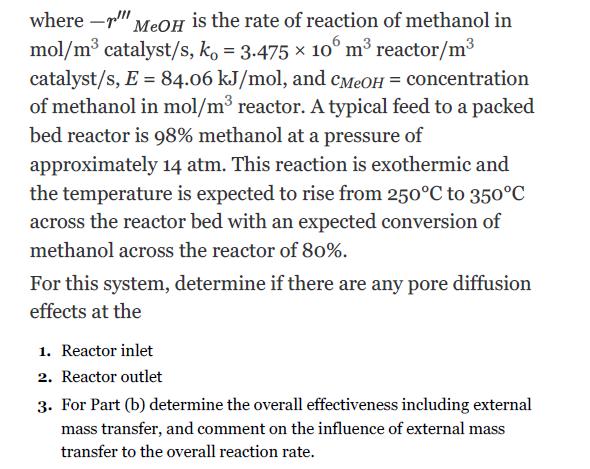
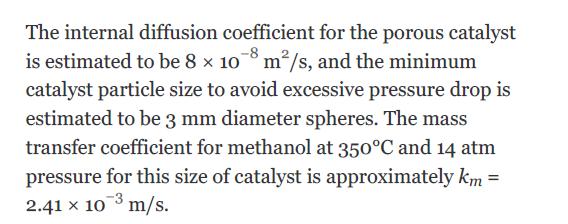
Using the data from Example 22.3, determine the volume of catalyst needed to convert 80% of a feed of pure methanol (at a rate of 12,500 kg/h) at 14 atm and 250°C in an adiabatic packed bed reactor for the following conditions:
1. When pore diffusion effects are ignored
2. Taking account of pore diffusion—note that the value of MT and the catalyst effectiveness should be evaluated along the length of the reactor![]()
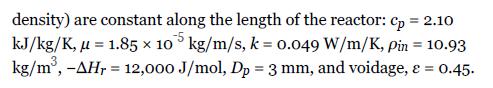
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting
Question Posted:





