Methanol can be produced from synthesis gas (syngas) by the following reaction: For the case when no
Question:
Methanol can be produced from synthesis gas (syngas) by the following reaction:![]()
For the case when no inerts are present and for a stoichiometric feed, the equilibrium expression has been determined to be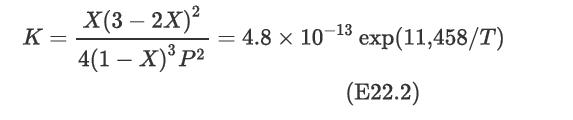 where X is the equilibrium conversion, P is the pressure in atmospheres, and T is the temperature in Kelvin.
where X is the equilibrium conversion, P is the pressure in atmospheres, and T is the temperature in Kelvin.
Construct a plot of equilibrium conversion versus temperature for four different pressures: 15 atm, 30 atm, 50 atm, and 100 atm. Interpret the significance of the results.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting
Question Posted:





