Repeat Problem 22.21 for a catalytic/enzymatic reaction with a rate expression of the form where c Ao
Question:
Repeat Problem 22.21 for a catalytic/enzymatic reaction with a rate expression of the form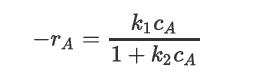
where cAo = 10 mol/L, k1 = 3.5 s-1, and k2 = 0.45 L/mol
Problem 22.21
In an isothermal batch reactor, a first-order, irreversible, liquid-phase reaction, A → B, occurs. The initial concentration is CAo.
1. By what percentage must the reaction time be increased to raise the conversion of A from 0.9 to 0.95?
2. If the initial conversion is 0.8, what will the final conversion be if the reaction time is increased by 50%?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting
Question Posted:





