An nth-order rate law is often used to model chemical reactions that solely depend on the concentration
Question:
An nth-order rate law is often used to model chemical reactions that solely depend on the concentration of a single reactant:
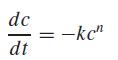
where c = concentration (mole), t = time (min), n = reaction order (dimensionless), and k = reaction rate (min−1 mole1−n). The differential method can be used to evaluate the parameters k and n. This involves applying a logarithmic transform to the rate law to yield,
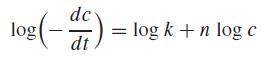
Therefore, if the nth-order rate law holds, a plot of the log(−dc/dt) versus log c should yield a straight line with a slope of n and an intercept of log k. Use the differential method and linear regression to determine k and n for the following data for the conversion of ammonium cyanate to urea:

Step by Step Answer:

Applied Numerical Methods With MATLAB For Engineers And Scientists
ISBN: 9781259027437
3rd Edition
Authors: Steven C. Chapra





