Assume that you have a solution of 0.1 M glucose 6-phosphate. To this solution you add the
Question:
Assume that you have a solution of 0.1 M glucose 6-phosphate. To this solution you add the enzyme phosphoglucomutase, which catalyzes the reaction:
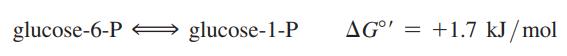
(a) Does this reaction proceed at all as written at 25 °C, and if so, what are the final concentrations of glucose 6-P and glucose 1-P?
(b) What effect would omitting the enzyme have on the reaction. Be specific.
(c) Under what cellular conditions, if any, would this reaction continuously produce glucose 1-P at a high rate?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Biochemistry Concepts And Connections
ISBN: 9780134641621
2nd Edition
Authors: Dean Appling, Spencer Anthony-Cahill, Christopher Mathews
Question Posted:





