Question: a. Calculate the de Broglie wavelength of the electron in the n = 1, 2, and 3 states of the hydrogen atom. Use the information
a. Calculate the de Broglie wavelength of the electron in the n = 1, 2, and 3 states of the hydrogen atom. Use the information in Table 38.2.
b. Show numerically that the circumference of the orbit for each of these stationary states is exactly equal to n de Broglie wavelengths.
c. Sketch the de Broglie standing wave for the n = 3 orbit.
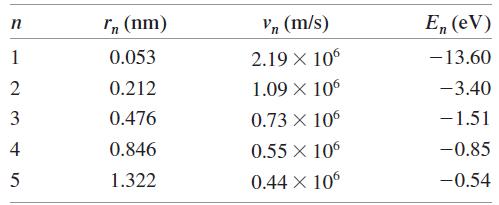
In (nm) Vn (m/s) E, (eV) 1 0.053 2.19 X 106 -13.60 2 0.212 1.09 X 106 -3.40 3 0.476 0.73 X 106 -1.51 4 0.846 0.55 X 10 -0.85 1.322 0.44 X 10 -0.54
Step by Step Solution
3.31 Rating (157 Votes )
There are 3 Steps involved in it
Solve a Using the data in Table 382 the wavelength of the electron in the n 1 state is Lik... View full answer

Get step-by-step solutions from verified subject matter experts


