The ideal gas law says that for n moles of an ideal gas, PV = nRT, where
Question:
The ideal gas law says that for n moles of an ideal gas, PV = nRT, where P is the pressure exerted by the gas, V is the volume of the gas, T is the temperature of the gas, and R is a constant (the gas constant). Compute the product
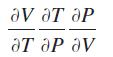
Transcribed Image Text:
Ꮩ ᎧᎢ ᎧᏢ ᎧᎢ ᎧᏢ Ꮩ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
PV nRT Solving for V nRT and P Sol...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Calculus For Business, Economics And The Social And Life Sciences
ISBN: 9780073532387
11th Brief Edition
Authors: Laurence Hoffmann, Gerald Bradley, David Sobecki, Michael Price
Question Posted:
Students also viewed these Mathematics questions
-
using Runge-kutta method of order four to Solve the initial value problem differential equation up to one approximation d?y dx2 +x?y dx with conditions y(0) = 1 and y'(0) = 0.5, at x = 0.1.
-
The ideal gas law relates the pressure P, volime V, and temperature T of an ideal gas: PV= nRT where " is the number of moles and R = 8.3145 J/(K. mol). Plots of pressure versus volume at constant...
-
The ideal gas law provides one way to estimate the pressure exerted by a gas in a container. The law is More accurate estimates can be made with the van der Waals equation where the term nb is a...
-
Concentric with the circle x 2 + y 2 + 2x 8y + 8 = 0 and passes through (2, 3)
-
On January 1, 2010, Bretz, Inc., acquired 60 percent of the outstanding shares of Keane Company for $573,000 in cash. The price paid was proportionate to Keanes total fair value although at the date...
-
A small object oscillates back and forth at the bottom of a frictionless hemispherical bowl, as the drawing illustrates. The radius of the bowl is R, and the angle u is small enough that the object...
-
ZeeZee's Construction Company has the opportunity to select one of four projects (A, B, C, or D) or choose the null (do-nothing) alternative. Each project requires a single initial investment and has...
-
(Basic Pension Worksheet) The following facts apply to the pension plan of Boudreau Inc. for the year 2010. Using the preceding data, compute pension expense for the year 2010, as part of your...
-
A firm just paid an annual dividend of $3.50 per share. Dividends are expected to grow at 5% per year. If the discount rate for this firm is 12%, how much is a share of this firm worth?Group of...
-
Determine the magnitude and direction, measured counterclockwise from the positive x' axis, of the resultant force of the three forces acting on the bracket. Given: F1 = 300 N F2 = 200 N F3 = 180 N...
-
Each of Exercises 81 through 85 involves either the chain rule for partial derivatives or the incremental approximation formula for functions of two variables. Use the formula obtained in Exercise 84...
-
A bicycle dealer has found that if 10-speed bicycles are sold for x dollars apiece and the price of gasoline is y cents per gallon, then approximately bicycles will be sold each month. If the price...
-
Using a situation that has not been discussed in the text, write a real-world problem that you think involves three variables that vary jointly. Exchange your problem with another students to solve...
-
Find f(x + h) - f(x) h f(x)=x-11 for the given function.
-
Organizational Hierarchies. Some theorists believe that computer-mediated communication will eventually eliminate the hierarchical structure of organizations, largely because CMC encourages...
-
A circular space station spins about its axis to simulate gravity in the living quarters located along its periphery. What is the direction of acceleration of a person standing at the periphery?
-
What best describes how a business analyst works with different levels of detail as they work? First, the Business Analyst analyzes details of how the technical systems work. They then identify the...
-
Question 5. [20 points] Business Process & Digital Transformation. Business processes are essential to businesses. With the movement towards digital transformation, business processes are greatly...
-
A study by researchers at the University of Maryland addressed the question of whether the mean body temperature of humans is 98.6F. The results of the study by P. Mackowiak et al. appeared in the...
-
Refrigerant R-12 at 30C, 0.75 MPa enters a steady flow device and exits at 30C, 100 kPa. Assume the process is isothermal and reversible. Find the change in availability of the refrigerant.
-
A survey of American consumers asked respondents to report the amount of money they spend on bakery products in a typical month. If we assume that the population standard deviation is $5, can we...
-
Many Americans contributed to their 401k investment accounts. An economist wanted to determine how well these investments performed. A random sample of Americans with 401k investments were surveyed...
-
A survey of 25- to 35-year-old Americans with professional or Ph.D. degrees was asked to report their monthly incomes. Can we conclude at the 10% significance level that the mean income exceeds...
-
Questions: 1. Briefly discuss the ethical implications of this case and COVID-19 essential workers. 2. End your paper with a conclusive paragraph that explains how the sections correspond to ethics...
-
Evaluate the decision made by Dr. Murphy and Educations Management Services (EMS) in terms of the process of going about the closure. Explain how the behavioral decision-making model was applied in...
-
What key issues and their implications are highlighted in this case (both financial and non-financial)? 2. What are the characteristics of the historical sales data? 3. What options/strategies are...

Study smarter with the SolutionInn App


