A clever chemical engineer has devised the thermally operated elevator shown in the accompanying diagram. The elevator
Question:
A clever chemical engineer has devised the thermally operated elevator shown in the accompanying diagram. The elevator compartment is made to rise by electrically heating the air contained in the pistonand-cylinder drive mechanism, and the elevator is lowered by opening a valve at the side of the cylinder, allowing the air in the cylinder to slowly escape. Once the elevator compartment is back to the lower level, a small pump forces out the air remaining in the cylinder and replaces it with air at 20◦C and a pressure just sufficient to support the elevator compartment. The cycle can then be repeated. There is no heat transfer between the piston, cylinder, and the gas; the weight of the piston, elevator, and elevator
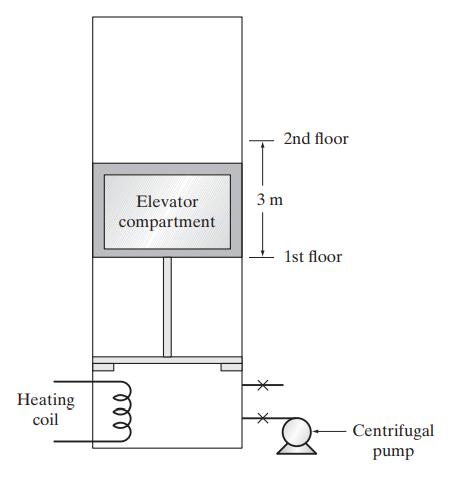
contents is 4000 kg; the piston has a surface area of 2.5 m2; and the volume contained in the cylinder when the elevator is at its lowest level is 25 m3. There is no friction between the piston and the cylinder, and the air in the cylinder is assumed to be an ideal gas with C∗P = 30 J/(mol K).
a. What is the pressure in the cylinder throughout the process?
b. How much heat must be added to the air during the process of raising the elevator 3 m, and what is the final temperature of the gas?
c. What fraction of the heat added is used in doing work, and what fraction is used in raising the temperature of the gas?
d. How many moles of air must be allowed to escape in order for the elevator to return to the lowest level?
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





