A gas is continuously passed through an adiabatic turbine at the rate of 2 mol/s. Its initial
Question:
A gas is continuously passed through an adiabatic turbine at the rate of 2 mol/s. Its initial temperature is 600 K, its initial pressure is 5 bar and its exiting pressure is 1 bar. Determine the maximum rate at which work can be obtained in this process. The gas is described by an augmented Clausius equation of state
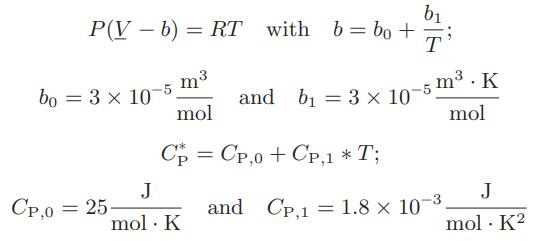
Transcribed Image Text:
b₁ P(V - b) = RT with b = bo+i T bo = 3 × 10-5 m3 mol Cp = Cp,o+Cp,1*T; CP,0 = 25 J mol. K and b₁ = 3 x 10-5 m³. K mol J mol. K² and CP,1 1.8 x 10-³. =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
To determine the maximum rate at which work can be obtained from the gas in an adiabatic turbine we ...View the full answer

Answered By

Atuga Nichasius
I am a Highly skilled Online Tutor has a Bachelor’s Degree in Engineering as well as seven years of experience tutoring students in high school, bachelors and post graduate levels. I have a solid understanding of all learning styles as well as using asynchronous online platforms for tutoring needs. I individualise tutoring for students according to content tutoring needs assessments.
My strengths include good understanding of all teaching methods and learning styles and I am able to convey material to students in an easy to understand manner. I can also assists students with homework questions and test preparation strategies and I am able to help students in math, gre, business , and statistics
I consider myself to have excellent interpersonal and assessment skills with strong teaching presentation verbal and written communication
I love tutoring. I love doing it. I find it intrinsically satisfying to see the light come on in a student's eyes.
My first math lesson that I taught was when I was 5. My neighbor, still in diapers, kept skipping 4 when counting from 1 to 10. I worked with him until he could get all 10 numbers in a row, and match them up with his fingers.
My students drastically improve under my tutelage, generally seeing a two grade level improvement (F to C, C to A, for example), and all of them get a much clearer understanding!
I am committed to helping my students get the top grades no matter the cost. I will take extra hours with you, repeat myself a thousand times if I have to and guide you to the best of my ability until you understand the concept that I'm teaching you.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
A gas is continuously passed through an adiabatic turbine at the rate of 2 mol/s. Its initial temperature is 600 K, its initial pressure is 5 bar and its exiting pressure is 1 bar. Determine the...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
An ideal gas expands in an adiabatic turbine from 1200 K, 600 kPa to 700 K. Determine the turbine inlet volume flow rate of the gas, in m3/s, required to produce turbine work output at the rate of...
-
Song Corp's stock price at the end of last year was $26.25 and its earnings per share for the year were $1.30. What was its P/E ratio?
-
Ryan Corporation manufactures and sells a variety of household cleaning products in interstate commerce. On national television, Ryan falsely advertises that its laundry liquid is biodegradable. Has...
-
Develop a formal proof of correctness for alpha-beta pruning. To do this, consider the situation shown in Figure. The question is whether to prune node nj, which is a max- node and a descendant of...
-
Thomas Rusnack and his then-wife, Analisa Rusnack, opened a home equity line of credit (HELOC) with Cardinal Bank in August 2003. Between 2003 and 2006, the Rusnacks periodically drew on the HELOC...
-
On June 10, Naveen Company purchased $8,000 of merchandise from Jarrah Company, FOB shipping point, terms 2/10, n/30. Naveen pays the freight costs of $400 on June 11. Damaged goods totaling $300 are...
-
By how much does the fluid level rise in the side of the manometer that is open to the atmosphere? By how much does the fluid level rise in the side of the manometer that is open to the atmosphere if...
-
Methane at 260 K is to be isothermally compressed from 0.1 MPa to 1.0 MPa. a. What is the minimum work required, and how much heat must be removed to keep the compression process isothermal? b. If...
-
In a continuous manufacturing process, chlorodifluoromethane (CHClF 2 ), initially at 10 bar and 420 K, passes through an adiabatic pressure-reducing valve so that its pressure drops to 0.1 bar (this...
-
The Gidget Widget Corporation produces widgets. The fixed expenses are $65,210, and the variable expenses are $4.22 per widget. Express the expense function algebraically.
-
Compare and contrast individualistic and collectivistic cultures. Explain your interpretation of what an individualistic culture is. Explain your interpretation of what a collectivist culture is. Do...
-
Find the domain of the function. 72 f(x) = x -9x-22 What is the domain of f? (Type your answer in interval notation.)
-
The Central Bank of Nigeria (CBN) has introduced a new policy on cash-based transactions which stipulates a cash handling charge on daily cash withdrawals that exceed N500,000 for Individuals and...
-
When it comes to credit history which statement is right. Your credit history is reported every 60 days. Any unpaid bills, late payments and bad debt write offs remain for 7 years and then disappear....
-
How do postmodern discourses on identity challenge traditional essentialist notions, and what implications do these challenges have for understanding the fluidity and multiplicity of individual and...
-
Because Landon Corporation (see Problem 19) is being pressured to complete the product development project at the earliest possible date, the project leader requested that the possibility of crashing...
-
Listed below are several terms and phrases associated with basic assumptions, broad accounting principles, and constraints. Pair each item from List A (by letter) with the item from List B that is...
-
Compute and plot the unit-step response of the following model. 10 + + 2 %3Df+ 7f
-
Find the reduced form of the following state model. -4 -1 2 -3 () 15
-
The following state model describes the motion of a certain mass connected to a spring, with viscous friction on the surface, where m = 1, c = 2, and k = 5. a. Use the initial function to plot the...
-
You set up a C&D debris program at your site and recycle all the cardboard, gypsum wallboard, steel, concrete and brick, and wood for a new construction project. Your dumpster count at the end of the...
-
Protrade Corporation acquired 80 percent of the outstanding voting stock of Seacraft Company on January 1, 2023, for $516,000 in cash and other consideration. At the acquisition date, Protrade...
-
You are redeveloping an acre of land that was originally an asphalt parking lot. Along with the building and parking, you plan to turn 10,000 ft2 of the lot into landscaped areas with natural and...

Study smarter with the SolutionInn App


