A gas mixture containing equimolar quantities of carbon dioxide and hydrogen is to be reformed by passing
Question:
A gas mixture containing equimolar quantities of carbon dioxide and hydrogen is to be “reformed” by passing it over a catalyst. The pressure in the reformer will be determined by the possibility of solid carbon deposition. Although a large number of reactions are possible, only the following are believed to occur:
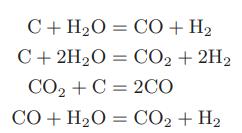
a. At temperatures between 600 and 1000 K, over what range of pressure will carbon deposit if each of the reactions is assumed to achieve equilibrium?
b. For this feed, what pressure should be maintained for exactly 30 percent of the carbon present in the feed to precipitate as solid carbon at each temperature between 600 and 1000 K?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





