A process is being developed to produce high-purity titanium. As part of the proposed process, titanium will
Question:
A process is being developed to produce high-purity titanium. As part of the proposed process, titanium will be kept in a quartz (silicon dioxide) crucible at 1273 K. A chemical engineer working on the process is concerned that the titanium could reduce the silicon dioxide, producing titanium dioxide and elemental silicon, which would lower the purity of the titanium. Is this concern justified?
Data: At 1273 K
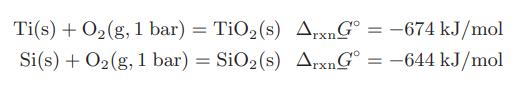
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





