A relatively simple cubic equation of state is where a is a constant. a. Determine the relationship
Question:
A relatively simple cubic equation of state is
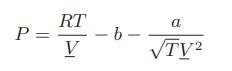
where a is a constant.
a. Determine the relationship between the a and b parameters in this equation of state and the critical temperature and pressure of the fluid.
b. Obtain an expression for the constant-volume heat capacity as a function of temperature, volume, the critical temperature and pressure, and the ideal heat capacity.
c. The constant-volume heat capacities of real fluids diverge as the critical point is approached. Does the constant-volume heat capacity obtained from this equation of state diverge as the critical point is approached?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





