An equimolar mixture of nitrogen and acetylene enters a steady-flow reactor at 25C and 1 bar of
Question:
An equimolar mixture of nitrogen and acetylene enters a steady-flow reactor at 25°C and 1 bar of pressure. The only reaction occurring is
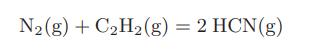
The product leaves the reactor at 600°C and contains 24.2 percent mole fraction of HCN. How much heat is supplied to the reactor per mole of HCN?
Transcribed Image Text:
N₂(g) + C₂H₂(g) = 2 HCN(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To calculate the heat supplied to the reactor per mole of HCN we need to perform an energy balance on the reactor The energy balance states that the t...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
An equimolar mixture of nitrogen and acetylene enters a steady-flow reactor at 25(C and atmospheric pressure. The only reaction occurring is: N2(g) + C2H2 ( 2HCN(g). The product gases leave the...
-
An equimolar mixture of oxygen and nitrogen enters a compressor operating at steady state at 10 bar, 220 K with a mass flow rate (m) of 1 kg/s. The mixture exits the compressor at 60 bar, 400 K with...
-
Jacky is single and will turn 24 this year. He doesn't have any dependents. He does not have any group coverage. He drives a motorcycle to work every day. From your perspective which insurance policy...
-
Pritchett Company reported the following year-end data: Cash $ 25,000 8,000 Short-term investments Accounts receivable (current) Inventory 19,500 27,500 Prepaid (current) assets 11,000 Total current...
-
Yolanda Christophe filed a bankruptcy petition under Chapter 13. Her scheduled debts consist of $11,100 of secured debt, $9,300 owed on an unsecured student loan, and $6,960 of other unsecured debt....
-
A certain area of the eastern United States is, on average, hit by 6 hurricanes a year. Find the probability that for a given year that area will be hit by (a) Fewer than 4 hurricanes; (b) Anywhere...
-
Andrew Reitz established a trust in 2000, naming his sons, James and John, as sole beneficiaries and himself as trustee. Upon Andrews death, Hal Rachal Jr., the attorney who drafted the trust, became...
-
The 2012 comparative balance sheet and income statement of Rolling Hills, Inc., follow: Additionally, Rolling Hills purchased land of $23,600 by financing it 100% with long-term notes payable during...
-
A bug sits on a record moving at a constant angular speed of 0 = 3.5 rad/s. Starting at t = 0 s, the record player is turned off and is given a constant angular acceleration of = 1.0 rad/s 2 . The...
-
The following data are available for the isothermal heat of mixing of trichloromethane (1) and ethanol (2) at 30C [reference: J. P. Shatas, M. M. Abbott, and H. C. Van Ness, J. Chem. Eng. Data, 20,...
-
Using the data below, calculate the partial molar enthalpies of 1-propanol and water as a function of composition at both 25C and 50C. data: V. P. Belousov, Vent. Leningrad Univ. Fiz., Khim, 16(1),...
-
You are in the market for a used car. At a used car lot, you know that the blue book value for the cars you are looking at is between $20,000 and $24,000. If you believe the dealer knows as much...
-
What's the present value of a $970 annuity payment over four years if interest rates are 8 percent? (Do not round intermediate calculations and round your final answer to 2 decimal places.) Present...
-
Identify the engineering discipline you want to pursue, then research that discipline. Explain what those engineers do. Explain why you would like to enter that discipline. Your response should be at...
-
$190 $264 $224 $240 What is Jamie Lee's passive income? Submit
-
You are a newly promoted manager at Standards & Co., a CPA firm, in their audit practice. During your training, you are instructed to select an example of filed annual financial statements (Form...
-
Approaching their tenth anniversary together, Dana and Sandy, have scheduled a meeting with their advisor to review and realign, if necessary, their current financial affairs. The Family The couple,...
-
Would process costing work well for a service firm? Why or why not?
-
(a) Given a mean free path = 0.4 nm and a mean speed vav = 1.17 105 m/s for the current flow in copper at a temperature of 300 K, calculate the classical value for the resistivity of copper. (b)...
-
Develop a table that lists advantages and disadvantages of the three design concepts for a mousetrap-powered vehicle in the case study in Section 2.4. Discuss the trade-offs between front and rear...
-
Three concepts for the drive mechanism in a mousetrap-powered vehicle are described in Section 2.4. Develop another concept, prepare several sketches, and write a brief description of it.
-
Express your weight in the units of pounds and newtons, and your mass in the units of slugs and kilograms.
-
Consider the following C code fragments and answer the associated questions. Assume that the code is running on a Nios II processor which is little endian. The Altera data types have been used which...
-
Write a VBA subroutine to compute the average molecular weight of a gas mixture (MW average) with the following composition by mass (mass of component/total mass). Recall: MWaverage = where yi is the...
-
The following "C" program (code fragment) is given - int i,j; if (i>j) { i= i+5; } else { i=0; j++; } Activities to do- 1. Provide the Y86 assembly language code for the "C" program. 2. Verify this...

Study smarter with the SolutionInn App


