At T = 60C the vapor pressure of methyl acetate is 1.126 bar, and the vapor pressure
Question:
At T = 60°C the vapor pressure of methyl acetate is 1.126 bar, and the vapor pressure of methanol is 0.847 bar. Their mixtures can be described by the oneconstant Margules equation
![]()
where R is the gas constant and T is temperature in K.
a. Plot the fugacity of methyl acetate and methanol in their mixtures as a function of composition at this temperature.
b. The Henry’s law coefficient Hi is given by the equation
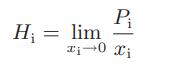
Develop an expression for the Henry’s law constant as a function of the A parameter in the Margules expression, the vapor pressure, and composition. Compare the hypothetical pure component fugacity based on the Henry’s law standard state with that for the usual pure component standard state.
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





