Bruno and coworkers [Int. J. Thermophys., 7, 1033 (1986)] describe an apparatus that contains a palladium-silver membrane
Question:
Bruno and coworkers [Int. J. Thermophys., 7, 1033 (1986)] describe an apparatus that contains a palladium-silver membrane that is permeable to hydrogen but not other gases. In their experiments after equilibration they measure the temperature T1 in the apparatus, the pressure P1 of pure hydrogen on one side of the semipermeable membrane, and the equilibrium composition of hydrogen xH2 and other gases and pressure P2 on the other side of the membrane. Next, by equating the hydrogen fugacities on both sides of the membrane,
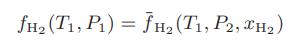
and calculating the fugacity of the pure hydrogen using known virial coefficients from
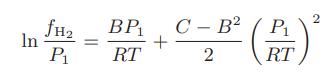
they have a direct measure of the fugacity of hydrogen in the mixture. Some of their results are given here:
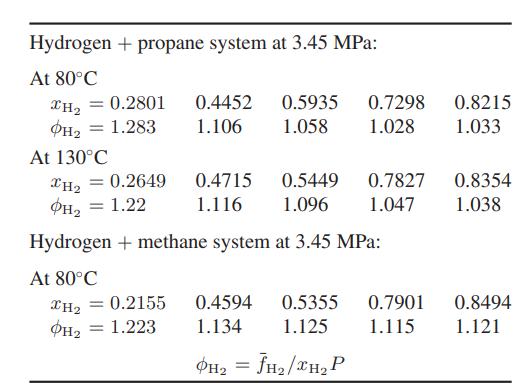
Compare these experimental results with the predicted fugacity coefficients for hydrogen in these mixtures calculated using the Peng-Robinson equation of state. Determine the sensitivity of the predictions to the value of the binary interaction parameter.
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





