For real gases the Joule-Thomson coefficient is greater than zero at low temperatures and less than zero
Question:
For real gases the Joule-Thomson coefficient is greater than zero at low temperatures and less than zero at high temperatures. The temperature at which μ is equal to zero at a given pressure is called the inversion temperature.
a. Show that the van der Waals equation of state exhibits this behavior, and develop an equation for the inversion temperature of this fluid as a function of its specific volume.
b. Show that the van der Waals prediction for the inversion temperature can be written in the corresponding-states form
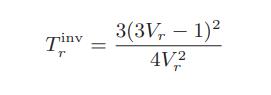
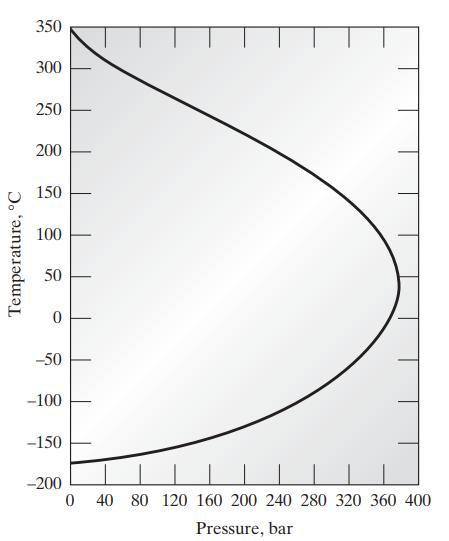
c. The following graph shows the inversion temperature of nitrogen as a function of pressure. Plot on this graph the van der Waals prediction for the inversion curve for nitrogen.
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler




