Isopropyl alcohol is to be dehydrogenated in the gas phase to form propionaldehyde according to the reaction
Question:
Isopropyl alcohol is to be dehydrogenated in the gas phase to form propionaldehyde according to the reaction
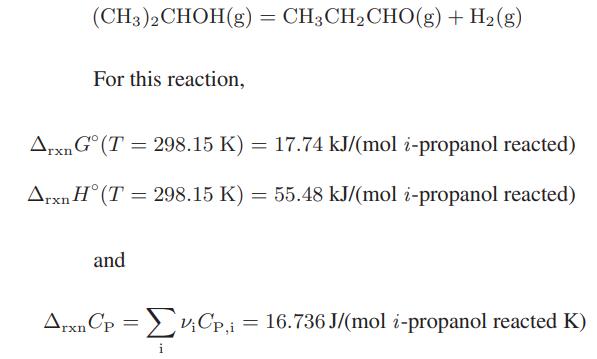
Compute the equilibrium fraction of isopropyl alcohol that would be dehydrogenated at 500 K and 1.013 bar.
Transcribed Image Text:
(CH3)2CHOH(g) = CH3 CH₂CHO(g) + H₂(g) For this reaction, Arxn Gᵒ (T = 298.15 K) = 17.74 kJ/(mol i-propanol reacted) Arxn H (T = 298.15 K) = 55.48 kJ/(mol i-propanol reacted) and ArxnCp = V₁Cp,i = 16.736 J/(mol i-propanol reacted K) ΣvCp, i
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
The information you sent shows the following reaction CH32CHOHg CH3CH2CHOg H2g This reaction is dehy...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
Redo Problem 13.1 using Aspen Plus. Problem 13.1 Isopropyl alcohol is to be dehydrogenated in the gas phase to form propionaldehyde according to the reaction Compute the equilibrium fraction of...
-
An electroplating process supplies a coating thickness of 800 nm. Calculate the area which would be covered using 1 kg of gold, mindful that the density of gold is 19300 kg/m3
-
The decomposition of iodoethane in the gas phase proceeds according to the following equation: C2H5I(g) C2H4(g) + HI(g) At 660. K, k = 7.2 10-4 s-1; at 720. K, k = 1.7 10-2 s-1. What is the rate...
-
In Exercises, find the limit. x-4 lim x-00x + 1
-
John Biggs and Patty Jorgenson are both cost accounting managers for a manufacturing division. During lunch yesterday, Patty told John that she was planning on quitting her job in three months...
-
Refer to the computer solution of the Kelson Sporting Equipment problem in Figure. THE MANAGEMENT SCIENTIST SOLUTION FOR THE KELSON SPORTING EQUIPMENT PROBLEM a. Determine the objective coefficient...
-
State a conclusion. Exercises 1015 refer to the following data: Anthropologists can estimate the birthrate of an ancient society by studying the age distribution of skeletons found in ancient...
-
On January 1, 2014, Plains Power Company overhauled four turbine engines that generate power for customers. The overhaul resulted in a slight increase in the capacity of the engines to produce power....
-
For its fiscal year ending October 31, 2022, Lily Corporation reports the following partial data. Income before income taxes $550,000 Income tax expense (25% x $421,000) 105,250 Income from...
-
Ionic liquids are salts with melting temperatures that are sufficiently low that they are liquids at or near room temperature. They consist of a larger cation and a smaller anion, for example,...
-
Carbon dioxide can react with graphite to form carbon monoxide, and the carbon monoxide formed can further react to form carbon and oxygen: Determine the equilibrium composition when pure carbon...
-
Discuss the assumptions and advantages of multistage compression.
-
(Enter accounts in ascending account order.) Revenue Sales revenue Less: Net sales revenue 70 Less: Gross profit Other revenue: Expenses Total expenses Net profit/(loss) List of Accounts Save for...
-
Jasmine is a large company which manufactures children's toys. It is a private UK limited company with a diversified shareholder base. The company has its headquarters in London, and as part of a...
-
Rose Hill, a soybean farm in northern Minnesota, has a herd of 4 5 dairy cows. The cows produce approximately 2 , 5 2 0 gallons of milk per week. The farm currently sells all its milk to a nearby...
-
1 Which root type is assigned to a bilateral mammogram procedure? 2 Which body system value and body part value are assigned to the following procedure: Body system: Body part: MRI OF THE PELVIS...
-
Production data: Kilograms in process, May 1 (materials 100% complete; conversion 80% complete) Kilograms started into production during May Kilograms completed and transferred to Coating Kilograms...
-
Barans Company currently has an average collection period of 55 days and annual sales of $1 billion. Assume a 365-day year. a. What is the firms average accounts receivable balance? b. If the...
-
What mass of KBr (in grams) should you use to make 350.0 mL of a 1.30 M KBr solution?
-
Determine the moment about point A of each of the three forces acting on the beam. F = 375 lb F = 500 lb . 0.5 ft 8 ft 6 ft 5ft- 5 ft- 30 F3 = 160 lb
-
Determine the moment about point B of each of the three forces acting on the beam. F = 375 lb F2 = 500 lb 3. B. 0.5 ft tontsa- 8 ft 6 ft -5 ft- 30 F3 = 160 lb
-
The crowbar is subjected to a vertical force of P = 25 lb at the grip, whereas it takes a force of F = 155 lb at the claw to pull the nail out. Find the moment of each force about point A and...
-
16. The row reduced echelon form of the real 4 6-matrix A is [10 2 0 1 0 1 -1 0 -2 0 R = 00 0 1 3 1 0 0 0 0 0 0 (a) Find a basis Bo for the row space of A and extend it to a basis B of R6. (b) If we...
-
"Paula is a social worker in a fostercare agency. She has been working with 9-year-old Rita, her foster mother, Flo, and biological mother, Stella, for three years. Rita was removed from her mother's...
-
You are 22 years old and currently have $100,000 in savings for retirement. You will start making annual contributions to your savings at the beginning of each year in the amount of $2,500. If you...

Study smarter with the SolutionInn App


