Ionic liquids are salts with melting temperatures that are sufficiently low that they are liquids at or
Question:
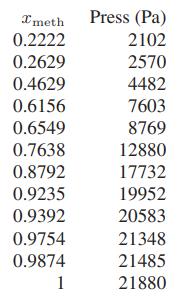
Ionic liquids are salts with melting temperatures that are sufficiently low that they are liquids at or near room temperature. They consist of a larger cation and a smaller anion, for example, 1-methyl-3- butylimidazolium octyl sulfate [BMIN][OctS], and it is because of their size and complex geometry that they do not crystallize easily and are liquids at room temperature. Also, ionic liquids have very low (essentially zero) vapor pressure, and therefore are of great interest as “green” solvents, since they are not lost into the atmosphere. In fact, we can consider [BMIN][OctS] to have zero vapor pressure. Below are data for the equilibrium pressure above mixtures of methanol and [BMIN][OctS] at 303.15 K from Safarov, Verevkin, Bich and Heintz (J. Chem. Eng. Data, 2006, 51, 518). Here xmeth is the mole fraction of methanol assuming the ionic liquid is not ionized (i.e., only the molecule, not anions and cations, are present).
a. Determine the activity coefficients of methanol as a function of concentration,
b. Determine the activity coefficients of [BMIN] [OctS] as a function of concentration.
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





