Many thermodynamic and statistical mechanical theories of fluids lead to predictions of the Helmholtz energy A with
Question:
Many thermodynamic and statistical mechanical theories of fluids lead to predictions of the Helmholtz energy A with T and V as the independent variables; that is, the result of the theory is an expression of the form A = A(T,V ). The following figure is a plot of A for one molecular species as a function of specific volume at constant temperature. The curve on the left has been calculated assuming the species is present as a liquid, and the curve on the right assuming the species is a gas.
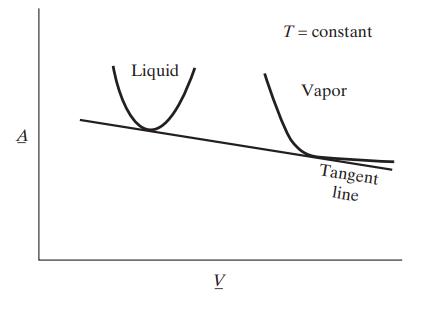
Prove that for the situation indicated in the figure, the vapor and liquid can coexist at equilibrium, that the specific volumes of the two coexisting phases are given by the points of tangency of the Helmholtz energy curves with the line that is tangent to both curves, and that the slope of this tangent line is equal to the negative of the equilibrium (vapor) pressure.
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





