The principle of corresponding states has far greater applicability in thermodynamics than was indicated in the discussion
Question:
The principle of corresponding states has far greater applicability in thermodynamics than was indicated in the discussion of Sec. 6.6. For example, it is possible to construct corresponding-states relations for both the vapor and liquid densities along the coexistence curve, and for the vapor pressure and enthalpy change on vaporization (ΔvapH) as a function of reduced temperature. This will be demonstrated here using the van der Waals equation as a model equation of state.
a. Show that at vapor-liquid equilibrium
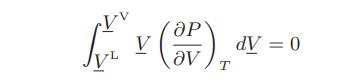
b. Show that the solution to this equation for the van der Waals fluid is
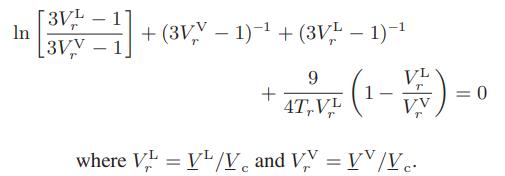
c. Construct a corresponding-states curve for the reduced vapor pressure Prvap of the van der Waals fluid as a function of its reduced temperature.
d. Describe how a corresponding-states curve for the enthalpy change on vaporization as a function of the reduced temperature can be obtained for the van der Waals fluid.
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





