One mole of carbon dioxide is to be compressed adiabatically from 1 bar and 25 C
Question:
One mole of carbon dioxide is to be compressed adiabatically from 1 bar and 25◦C to 10 bar. Because of irreversibilities and poor design of the compressor, the compressor work required is found to be 25 percent greater than that for a well-designed (reversible) compressor. Compute the outlet temperature of the carbon dioxide and the work that must be supplied to the compressor for both the reversible and irreversible compressors for the two cases below.
a. Carbon dioxide is an ideal gas with a constantpressure heat capacity of 37.151 J/(mol K).
b. Carbon dioxide is an ideal gas with the constantpressure heat capacity given in Appendix A.II.
Appendix A.II
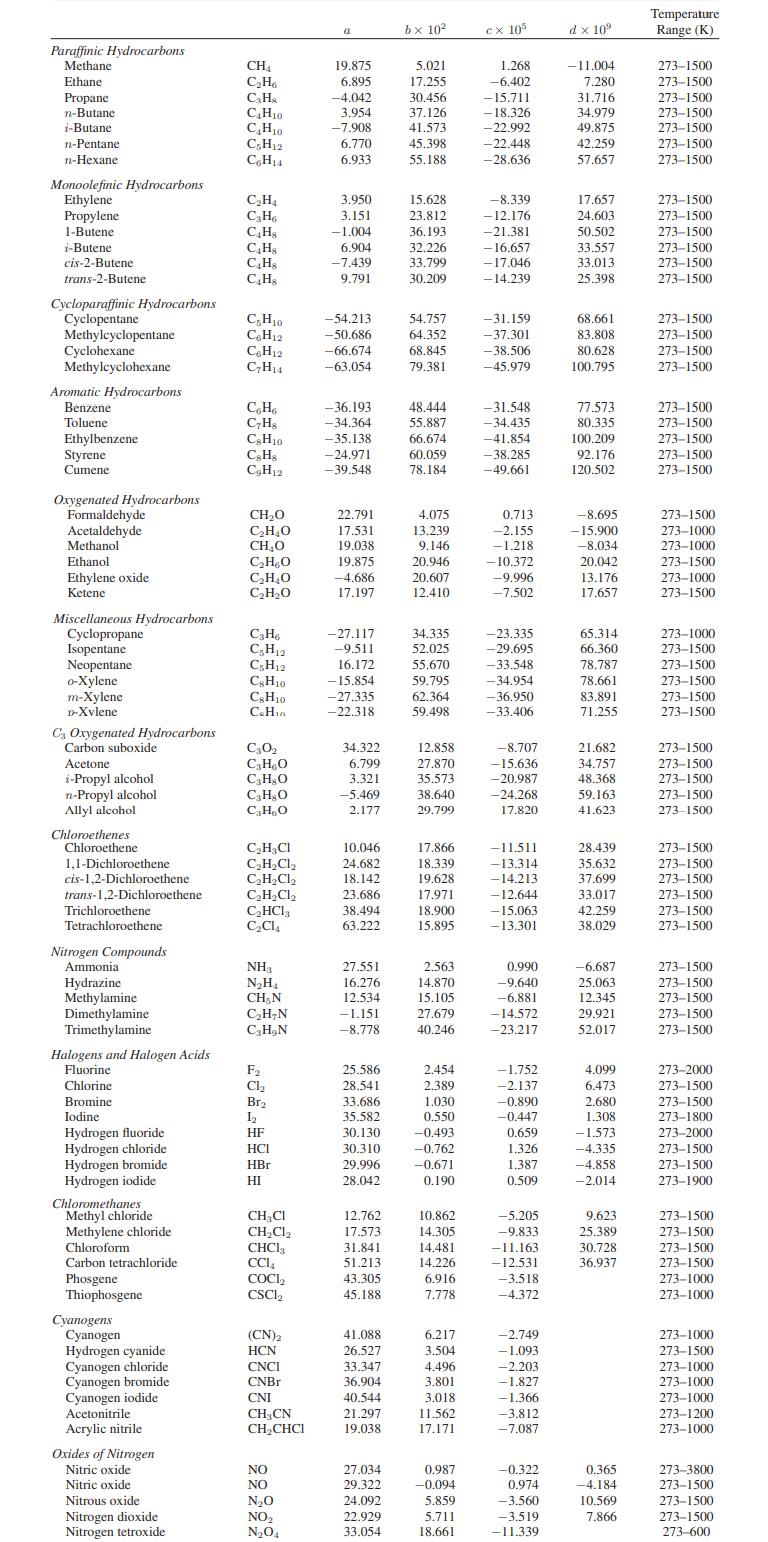
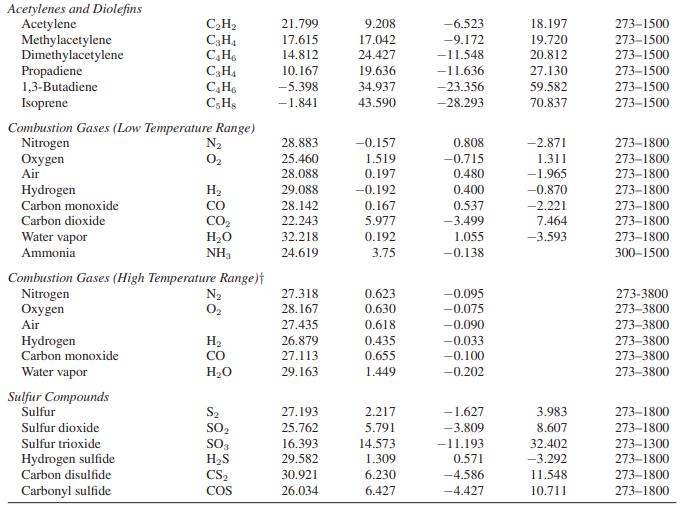
Paraffinic Hydrocarbons Methane Ethane Propane n-Butane i-Butane n-Pentane n-Hexane Monoolefinic Hydrocarbons Ethylene Propylene 1-Butene i-Butene cis-2-Butene trans-2-Butene Cycloparaffinic Hydrocarbons Cyclopentane Methylcyclopentane Cyclohexane Methylcyclohexane Aromatic Hydrocarbons Benzene Toluene Ethylbenzene Styrene Cumene Oxygenated Hydrocarbons Formaldehyde Acetaldehyde Methanol Ethanol Ethylene oxide Ketene Miscellaneous Hydrocarbons Cyclopropane Isopentane Neopentane o-Xylene m-Xylene D-Xvlene C3 Oxygenated Hydrocarbons Carbon suboxide Acetone i-Propyl alcohol n-Propyl alcohol Allyl alcohol Chloroethenes Chloroethene 1,1-Dichloroethene cis-1,2-Dichloroethene trans-1,2-Dichloroethene Trichloroethene Tetrachloroethene. Nitrogen Compounds Ammonial Hydrazine Methylamine Dimethylamine Trimethylamine Halogens and Halogen Acids Fluorine Chlorine Bromine Iodine Hydrogen fluoride Hydrogen chloride Hydrogen bromide Hydrogen iodide Chloromethanes Methyl chloride Methylene chloride Chloroform Carbon tetrachloride Phosgene Thiophosgene Cyanogens Cyanogen Hydrogen cyanide Cyanogen chloride Cyanogen bromide Cyanogen iodide Acetonitrile Acrylic nitrile Oxides of Nitrogen Nitric oxide Nitric oxide Nitrous oxide Nitrogen dioxide Nitrogen tetroxide CH₁ C₂H6 C₂H₂ C4H10 C₂H₁0 C5H12 C6H14 C₂H₁ C₂H6 C4H8 C4H8 C4Hs C4H8 C5H10 C6H12 C6H12 C₂H14 C6H6 C₂H8 CH10 CsH8 C₂H12 CH₂O C₂H₂O CH₂O C₂HBO C₂H₂O C₂H₂O C3H6 C₂H12 C5H12 CH10 C&H10 C.Hin C30₂ C3H₂O C3H8O C₂H₂O CHO C₂H₂Cl C₂H₂Cl₂ C₂H₂Cl₂ C₂H₂Cl₂ C₂HCl3 C₂C14 NH3 N₂H₁ CH, N C₂H₂N C₂H₂N F₂ Cl₂ Br₂ 1₂ HF HCI HBr HI CH₂Cl CH₂Cl₂ CHC13 CC14 COCI₂ CSCL (CN)2 HCN CNCI CNBr CNI CH3CN CH₂CHCI NO NO N₂O NO₂ N₂O4 a 19.875 6.895 -4.042 3.954 -7.908 6.770 6.933 3.950 3.151 -1.004 6.904 -7.439 9.791 -54.213 -50.686 -66.674 -63.054 -36.193 -34.364 -35.138 -24.971 -39.548 22.791 17.531 19.038 19.875 -4.686 17.197 -27.117 -9.511 16.172 -15.854 -27.335 -22.318 34.322 6.799 3.321 -5.469 2.177 10.046 24.682 18.142 23.686 38.494 63.222 27.551 16.276 12.534 -1.151 -8.778 25.586 28.541 33.686 35.582 30.130 30.310 29.996 28.042 12.762 17.573 31.841 51.213 43.305 45.188 41.088 26.527 33.347 36.904 40.544 21.297 19.038 27.034 29.322 24.092 22.929 33.054 bx 10² 5.021 17.255 30.456 37.126 41.573 45.398 55.188 15.628 23.812 36.193 32.226 33.799 30.209 54.757 64.352 68.845 79.381 48.444 55.887 66.674 60.059 78.184 4.075 13.239 9.146 20.946 20.607 12.410 34.335 52.025 55.670 59.795 62.364 59.498 12.858 27.870 35.573 38.640 29.799 17.866 18.339 19.628 17.971 18.900 15.895 2.563 14.870 15.105 27.679 40.246 2.454 2.389 1.030 0.550 -0.493 -0.762 -0.671 0.190 10.862 14.305 14.481 14.226 6.916 7.778 6.217 3.504 4.496 3.801 3,018 11.562 17.171 0.987 -0.094 5.859 5.711 18.661 cx 105 1.268 -6.402 -15.711 -18.326 -22.992 -22.448 -28.636 -8.339 -12.176 -21.381 -16.657 -17.046 -14.239 -31.159 -37.301 -38.506 -45.979 -31.548 -34.435 -41.854 -38.285 -49.661 0.713 -2.155 -1.218 -10.372 -9.996 -7.502 -23.335 -29.695 -33.548 -34.954 -36.950 -33.406 -8.707 -15.636 -20.987 -24.268 17.820 -11.511 -13.314 -14.213 -12.644 -15.063 -13.301 0.990 -9.640 -6.881 -14.572 -23.217 -1.752 -2.137 -0.890 -0.447 0.659 1.326 1.387 0.509 -5.205 -9.833 -11.163 -12.531 -3.518 -4.372 -2.749 -1.093 -2.203 -1.827 -1.366 -3.812 -7.087 -0.322 0.974 -3.560 -3.519 -11.339 d x 10⁹ -11.004 7.280 31.716 34.979 49.875 42.259 57.657 17.657 24.603 50.502 33.557 33.013 25.398 68.661 83.808 80.628 100.795 77.573 80.335 100.209 92.176 120.502 -8.695 -15.900 -8.034 20.042 13.176 17.657 65.314 66.360 78.787 78.661 83.891 71.255 21.682 34.757 48.368 59.163 41.623 28.439 35.632 37.699 33.017 42.259 38.029 -6.687 25.063 12.345 29.921 52.017 4.099 6.473 2.680 1.308 -1.573 -4.335 -4.858 -2.014 9.623 25.389 30.728 36.937 0.365 -4.184 10.569 7.866 Temperature Range (K) 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1000 273-1000 273-1500 273-1000 273-1500 273-1000 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-2000 273-1500 273-1500 273-1800 273-2000 273-1500 273-1500 273-1900 273-1500 273-1500 273-1500 273-1500 273-1000 273-1000 273-1000 273-1500 273-1000 273-1000 273-1000 273-1200 273-1000 273-3800 273-1500 273-1500 273-1500 273-600
Step by Step Answer:



Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Students also viewed these Engineering questions
-
Three points X1, X2, X3 are selected at random on a line L. What is the prob- ability X2 lies between X1 and X3?
-
A natural gas stream (essentially pure methane) is available at 310 K and 14 bar. The gas is to be compressed to 345 bar before transmission by underground pipeline. If the compression is carried out...
-
The molar heat capacities for carbon dioxide at 298.0 K are Cv = 28.95 J K-1 mol-1 Cp = 37.27 J K-1mol-1 The molar entropy of carbon dioxide gas at 298.0 K and 1.000 atm is 213.64 JK-1mol-1. a....
-
If you were advising a legal team on how to prepare for a discovery conference, what would you recommend or include? What issues should a legal team and its client consider? What are some of the...
-
Hanover leased a portion of his farm to Brown and Black, doing business as the Colorite Hatchery. Brown went upon the premises to remove certain chicken sheds that he and Black had placed there for...
-
Petty Cash, Bank Reconciliation Bill Jovi is reviewing the cash accounting for Nott leman, Inc., a local mailing service. Jovi?s review will focus on the petty cash account and the bank...
-
In spring 1989, Michael Jordan and the Chicago Bulls were in Indianapolis, Indiana, to play against the Indiana Pacers. At the same time, Karla Knafel was singing with a band at a hotel in...
-
Shaw Company sells goods that cost $300,000 to Ricard Company for $410,000 on January 2, 2012. The sales price includes an installation fee, which is valued at $40,000. The fair value of the goods is...
-
(a) Determine whether the money multiplier will increase or decrease following an increase in each of the following ratios. No explanation is required. [3 Points; 1 Point each] (i) The required...
-
If it is necessary to compress hydrogen to a higher pressure than is possible with the single-compression step above, an alternative is to use two compressors (or a two-stage compressor) with...
-
Hydrogen has an auto-ignition temperature of 853 K; that is, hydrogen will ignite spontaneously at that temperature if exposed to oxygen. Hydrogen is to be adiabatically and reversibly compressed...
-
In generating electricity, how do the environmental effects of coal combustion and conventional nuclear fission compare?
-
What do the visuals say about the problem you are trying to address in the automotive industry?
-
1) What is meant by testing a hypothesis? 2) What is meant by type I and type II errors? 3) What is meant by the level of significance? The level of confidence?
-
What I find more complex and difficult to model is possibility of making decision that will always produce the intended results, or offer you precise values of short and long-term gains. Do you think...
-
Why does the optimal consumption bundle occur when the budget line is tangent to the indifference curve? Assume that the indifference curves are convex?
-
what are ASEAN key's policies regulation relating to digital trade? how do regulated digital work in ASEAN? where are the regulatory challenges and fitfalls?
-
Parts III, V, and VI of this case study dealt with obtaining an understanding of internal control and assessing control risk for transactions affecting accounts payable of Pinnacle Manufacturing. In...
-
Discuss whether responsible human resources management should apply different standards for the home company and suppliers, for developed countries and developing countries, and for large companies...
-
A 10 m 8 m mat foundation is to be placed at 3 m depth in a saturated clay where c u = 60 kN/m 2 and = 0. Determine the net ultimate bearing capacity.
-
A 10 m 6 m mat foundation is placed at 2.0 m depth in sand where the average value of N 60 is 23. Determine the allowable net pressure that would limit the settlement to 75 mm, using Eqs. (9.47) and...
-
A 12 m 9 m mat foundation is placed at a depth of 3 m within sand where N 60 is 20. Using Eq. (10.14), estimate the elastic settlement when the net applied pressure is 250 kN/m 2 . Eqs. (10.14) Se...
-
s Retained earnings, December 31, 2021 Cost of buildings purchased during 2022 Net loss for the year ended December 31, 2022 Dividends declared and paid in 2022 Increase in cash balance from January...
-
Compare/Contrast features of all servers of Microsoft Windows to date. Please develop a comparison/contrast table in a Word document.
-
3. Wireless should not be used in mission critical situations unless there is either a backup network or somewhat costly and extensive security (including intrusion detection and rogue signal...

Study smarter with the SolutionInn App


