Redo Problem 6.22 with the SoaveRedlich-Kwong equation of state. Problem 6.22 A tank containing carbon dioxide at
Question:
Redo Problem 6.22 with the Soave–Redlich-Kwong equation of state.
Problem 6.22
A tank containing carbon dioxide at 400 K and 50 bar is vented until the temperature in the tank falls to 300 K. Assuming there is no heat transfer between the gas and the tank, find the pressure in the tank at the end of the venting process and the fraction of the initial mass of gas remaining in the tank for each of the following cases.
a. The equation of state of carbon dioxide is P(V − b) = RT with b = 0.0441 m3/kmol
b. Carbon dioxide obeys the law of corresponding states of Sec. 6.6.
c. Carbon dioxide obeys the Peng-Robinson equation of state. The low-pressure (ideal gas) heat capacity of CO2 is given in Appendix A.II.
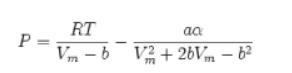
Appendix A.II
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





