The calcination of sodium bicarbonate takes place according to the reaction When this reaction was run in
Question:
The calcination of sodium bicarbonate takes place according to the reaction
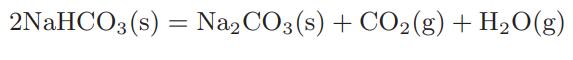
When this reaction was run in the laboratory by placing sodium bicarbonate in an initially evacuated cylinder, it was observed that the equilibrium total pressure was 0.826 kPa at 30°C and 166.97 kPa at 110°C. The heat of reaction for the calcination can be assumed to be independent of temperature.
a. What is the heat of reaction for this reaction?
b. Develop an equation for the equilibrium constant at any temperature.
c. At what temperature will the partial pressure of carbon dioxide in the reaction vessel be exactly 1 bar?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





