The data below are for the activity coefficients of lithium bromide in aqueous solutions as a function
Question:
The data below are for the activity coefficients of lithium bromide in aqueous solutions as a function of molality at 25°C
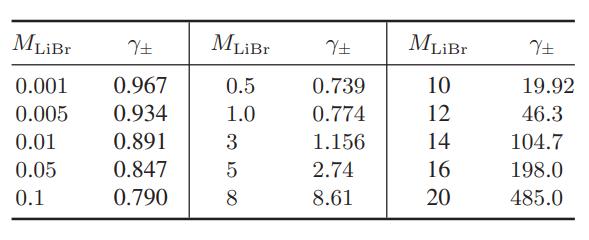
Compare the predictions of the Debye-Huckel model (Eqs. 9.10-15 and 9.10-16), and the extended DebyeHuckel models (Eqs. 9.10-17 and 9.10-18) with these data.


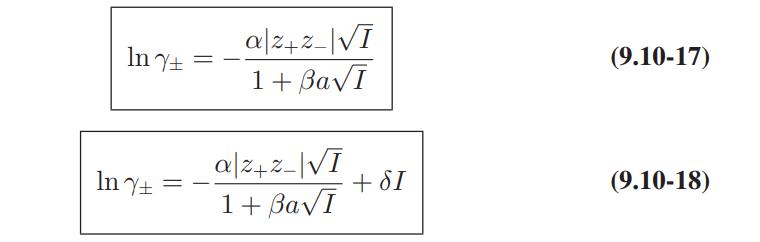
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





