The following data are available for mean activity coefficients of single electrolytes in water at 25C. Compare
Question:
The following data are available for mean activity coefficients of single electrolytes in water at 25°C.
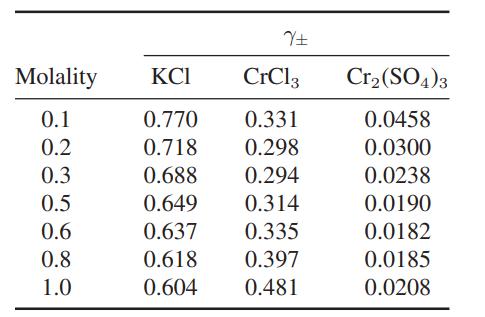
Compare these data with the predictions of the DebyeHuckel limiting law, Eq. 9.10-15, and Eq. 9.10-18 with βa = 1 and δ = 0.1|z+z−|.

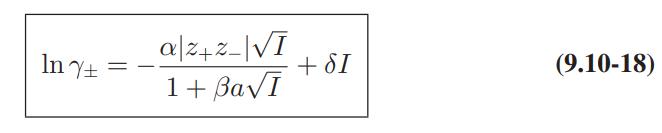
Transcribed Image Text:
Molality КСІ 0.1 0.770 0.2 0.718 0.3 0.688 0.5 0.649 0.6 0.8 1.0 0.637 0.618 0.604 Y+ CrCl3 0.331 0.298 0.294 0.314 0.335 0.397 0.481 Cr₂(SO4)3 0.0458 0.0300 0.0238 0.0190 0.0182 0.0185 0.0208
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To compare the given data with the predictions of the DebyeHckel limiting law we can use the activi...View the full answer

Answered By

Rayan Gilbert
I have been teaching since I started my graduation 3 years ago. As a student, working as Teacher/PA has been tough but made me learn the needs for student and how to help them resolve their problems efficiently. I feel good to be able to help out students because I'm passionate about teaching. My motto for teaching is to convey the knowledge I have to students in a way that makes them understand it without breaking a sweat.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
The following data are from Gennings, Chinchilli, and Carter, (1989). An in vitro toxicity study of isolated hepatocyte suspensions was conducted to study the impact of combining carbon tetrachloride...
-
The following data are available for three companies at the end of their fiscal years: Required Determine the amounts indicated by question marks. Company A $ 600,000 Finished goods, January 1 Cost...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
1. Prepare a schedule of cost of goods manufactured for Denim Bones for the year ended December 31, 2024. 2. Prepare an income statement for Denim Bones for the year ended December 31, 2024. 3. How...
-
Refer to the Nike information in Exhibit 4-5. Using the data for fiscal year 2001, compute both the gross profit and the gross profit percentage.
-
If deadlock is avoided by deadlock avoidance schemes, is starvation still possible? Explain your answer.
-
Choose a country from three of the regions presented in Table 6.7. Using the Internet, collect as much information as you believe is needed to identify the potential for market segments based on age,...
-
Upton Computers makes bulk purchases of small computers, stocks them in conveniently located warehouses, and ships them to its chain of retail stores. Upton's balance sheet as of December 31, 2004,...
-
X The function h is defined as h(x)=4-x2 x ln x, x>1 jh(x) Show that h(x) dx = 4ln 2- -1 15 16
-
The data below are for the activity coefficients of lithium bromide in aqueous solutions as a function of molality at 25C Compare the predictions of the Debye-Huckel model (Eqs. 9.10-15 and 9.10-16),...
-
There are several possible expressions that can be used for the Gibbs excess energy. One is the Redlich-Kister expansion where B = 0, but A and C are nonzero. Find expressions for the activity...
-
A sales manager for a major pharmaceutical company analyzes last years sales data for her 96 sales representatives, grouping them by region (1 = East Coast United States; 2 = Mid West United States;...
-
Lansing Company's current-year income statement and selected balance sheet data at December 31 of the current and prior years follow. LANSING COMPANY Income Statement For Current Year Ended December...
-
Name two works of art viewed in your virtual tour and explain what subjects, themes, and stylistic characteristics enhanced your appreciation of art. Describe the level of enjoyment the experience...
-
Identify the statement that is correct as it relates to the CVP income statement. It is part of the accounting information provided to all financial statement users. It is used internally by...
-
Western Led, recorded revenue for a five year construction contract evenly over the five years. describe Qualitative characteristic of financial statement foundation.
-
Who invest not less than US$500,000 in the Multi Facility Economic Zones (MFEZ) or a priority sector or product under the ZDA Act, are entitled to one of the following fiscal incentives?
-
What is the purpose of establishing a sinking fund?
-
The following processes constitute the air-standard Diesel cycle: 12: isentropic compression,23: constant-volume energy addition (T and P increase),34: constant-pressure energy addition (v...
-
Refer to the Fourier transform infrared spectrum in Figure 19-32. (a) The interferogram was sampled at retardation intervals of 1.2660 10 -4 cm. What is the theoretical wavenumber range (0 to ?) of...
-
The table shows signal-to-noise ratios recorded in a nuclear magnetic resonance experiment. Construct graphs of (a) signal-tonoise ratio versus n and (b) signal-to-noise ratio versus n, where n is...
-
Would you use a tungsten or a deuterium lamp as a source of 300-nm radiation? What kind of lamp provides radiation at 4-m wavelength?
-
Test marketing is a step in the new product development process. In the chapter it was stated that some marketers see test marketing as an essential step, almost a mandatory step. Other marketers see...
-
What does it take to create completely new innovative organizations, and what are some examples of these?
-
the formula to compute the budgeted direct labor cost is Multiple choice question. units to produce times direct labor required per unit divided by direct labor cost per hour units to produce times...

Study smarter with the SolutionInn App


